Key Points
Nearly 80% of the warfarin-treated patients with ICH had an INR within or below therapeutic range around 2 weeks before the event.
We can reduce ICH by using apixaban rather than warfarin and by avoiding concomitant aspirin, especially in patients with older age.
Abstract
We investigated the frequency and characteristics of intracranial hemorrhage (ICH), the factors associated with the risk of ICH, and outcomes post-ICH overall and by randomized treatment. We identified patients with ICH from the overall trial population enrolled in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial who received ≥1 dose of the study drug (n = 18 140). ICH was adjudicated by a central committee. Cox regression models were used to identify factors associated with ICH. ICH occurred in 174 patients; most ICH events were spontaneous (71.7%) versus traumatic (28.3%). Apixaban resulted in significantly less ICH (0.33% per year), regardless of type and location, than warfarin (0.80% per year). Independent factors associated with increased risk of ICH were enrollment in Asia or Latin America, older age, prior stroke/transient ischemic attack, and aspirin use at baseline. Among warfarin-treated patients, the median (25th, 75th percentiles) time from most recent international normalized ratio (INR) to ICH was 13 days (6, 21 days). Median INR prior to ICH was 2.6 (2.1, 3.0); 78.5% of patients had a pre-ICH INR <3.0. After ICH, the modified Rankin scale score at discharge was ≥4 in 55.7% of patients, and the overall mortality rate at 30 days was 43.3% with no difference between apixaban- and warfarin-treated patients. ICH occurred at a rate of 0.80% per year with warfarin regardless of INR control and at a rate of 0.33% per year with apixaban and was associated with high short-term morbidity and mortality. This highlights the clinical relevance of reducing ICH by using apixaban rather than warfarin and avoiding concomitant aspirin, especially in patients of older age. This trial was registered at www.clinicaltrials.gov as #NCT00412984.
Introduction
Intracranial hemorrhage (ICH) is one of the most feared and devastating complications of oral anticoagulation therapy for patients with atrial fibrillation (AF) and is associated with significant morbidity and mortality.1-3 Prior studies have reported mortality rates between 20% and 55% following anticoagulation-associated ICH.4-7 In the trials studying non–vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in patients with AF, 30-day mortality after ICH ranged from 36% to 43%.8-10
Apixaban, a direct factor Xa inhibitor, caused 58% less ICH than warfarin in patients with nonvalvular AF in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial.11 In the present study, we investigate the (1) frequency and characteristics of patients with ICH compared with those who did not present with ICH; (2) treatment effects of apixaban versus warfarin according to ICH type and location; (3) factors associated with the risk of ICH; (4) management of ICH in patients receiving oral anticoagulant therapy; and (5) outcomes following ICH overall and by treatment assignment (warfarin or apixaban).
Materials and methods
Patients and study design
The design and results of the ARISTOTLE trial have been previously published.11,12 In summary, 18 201 patients with nonvalvular AF and at least 1 additional risk factor for stroke (age ≥ 75 years, previous stroke/transient ischemic attack [TIA], symptomatic heart failure or left ventricular ejection fraction <40%, diabetes, or hypertension) were randomized to receive either 5 mg apixaban twice daily or dose-adjusted warfarin with a target international normalized ratio (INR) of 2.0 to 3.0. A reduced dose of apixaban (2.5 mg twice daily) was given to patients with ≥2 of the following characteristics: age ≥ 80 years, body weight ≤ 60 kg, and creatinine ≥ 1.5 mg/dL. Key exclusion criteria included clinically significant mitral stenosis, prosthetic mechanical heart valve, previous intracranial bleeding, severe renal insufficiency, recent stroke (within 7 days before randomization), and need for dual antiplatelet therapy. In this analysis, we included all patients who received at least 1 dose of the study drug (n = 18 140). Patients with ICH, as well as those without ICH, were followed until death or the end of the study, regardless of hospitalization, even if the study drug was interrupted. Appropriate ethics committees at participating sites approved the protocol, and all patients provided written informed consent.
ICH events
All ICH events were identified by site investigators and systematically adjudicated by a central committee, unaware of treatment assignment, using prespecified criteria. For ICH to be classified as a hemorrhagic stroke, the patient had to present with a nontraumatic abrupt onset focal neurologic deficit lasting at least 24 hours and a corresponding imaging modality confirming ICH. Therefore, all hemorrhagic strokes were counted as ICH, but not all ICH events met the criteria for hemorrhagic stroke.
The present analysis uses data from a supplemental case report form that collected additional details regarding ICH events (supplemental Appendix, available on the Blood Web site) using records collected during the adjudication process. Two independent physicians (P.O.G. and B.J.K.) reviewed each ICH case, and extracted details including ICH location, method of diagnosis, symptoms, type of event (spontaneous or traumatic), and treatment. The modified Rankin scale at discharge was determined retrospectively by a board-certified neurologist (B.J.K.) based on hospitalization reports. In cases of disagreement, a committee of 3 physicians (P.O.G., B.J.K., and R.D.L.) evaluated the ICH event and resolved the disagreement by consensus. For the final data set, patient-level data from the supplemental ICH case report form were combined with the main trial database.
ICH events were classified as intraparenchymal, subdural hematomas, or subarachnoid hemorrhages based on imaging reports. If a patient had multiple sites of ICH, the location judged most likely to be the origin and the most important component of ICH were defined by a neurologist (B.J.K.) based on the characteristics of the clinical presentation, imaging, and autopsy reports. The ICH event was considered traumatic when trauma was clearly stated in the patient report and the ICH was considered to be attributable to the trauma episode, regardless of the time interval between the trauma and the ICH event. If the patient presented with syncope followed by a fall, and the syncope was clinically judged to be part of the symptoms of the ICH, this event was then classified as spontaneous.
Statistical analysis
Baseline characteristics are presented for patients without ICH, with ICH overall, and by main location of bleeding. Categorical variables are presented as frequencies (percentages) and were compared using the Pearson χ2 test or exact test when appropriate. Continuous variables are summarized with medians (25th, 75th percentiles) and compared using the Wilcoxon rank-sum test. Cause-specific proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) comparing apixaban versus warfarin for intracranial bleeding by type and location.
A Cox proportional hazards model was used to identify predictors of ICH. Candidate variables for inclusion were: age, race, sex, region of enrollment, hypertension, coronary artery disease, history of stroke/TIA, history of bleeding, history of anemia, history of fall, liver disease, alcohol use, systolic blood pressure, diastolic blood pressure, weight, creatinine clearance, hemoglobin, platelets, serum albumin, prior use of vitamin K antagonist, aspirin use at randomization, use of nonsteroidal anti-inflammatory drugs, and randomized treatment. Continuous variables were tested for linearity, and nonlinear terms were used if needed. The proportional hazards assumption was tested for all candidate variables, and model selection was based on a backward selection algorithm with α-level to stay in the model set to 0.05. Variables selected in the final model are presented as χ2 and P values, along with HRs and 95% CIs. All tests were 2-sided, and P < .05 was considered statistically significant. Analysis was performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) at the Duke Clinical Research Institute (Durham, NC).
Results
ICH characteristics
Overall, the occurrence of ICH was 0.57 per 100 patient-years of follow-up (n = 174). No patient had >1 ICH event during the study period. Among those with ICH, the median time to ICH was 312 days (116, 481 days). The median time to ICH was 348 days (41, 563 days) among those randomized to apixaban and 279 days (113, 455 days) among those randomized to warfarin. Information about ICH location and type or diagnosis method was missing for 11 and 8 patients, respectively.
In most patients, ICH was identified by at least 1 imaging study: computerized tomography (143 [86.2%] patients), magnetic resonance imaging (5 [3.0%] patients), or both (10 [6.0%] patients). Only 3 patients were diagnosed by autopsy (1.8%), 4 had an autopsy confirming ICH after undergoing an imaging study (2.4%), and 1 was diagnosed by cerebral spinal fluid (0.6%). The main locations of ICH were intraparenchymal (105 [64.4%] patients), subdural (44 [27.0%] patients), and subarachnoid (14 [8.6%] patients). Overall, 19 patients presented with >1 site of ICH; of those, 11 were traumatic. Among those with intraparenchymal ICH, 6 presented with only intraventricular bleeding. The majority of ICHs was spontaneous (119 [71.7%]), and 47 (28.3%) were traumatic. Location of ICH was not available for 3 patients with spontaneous ICH. Most subdural hematomas were traumatic (65.9%) (Table 1). More than half of the subarachnoid bleeding events were spontaneous (64.3%), and only 12.4% of the intraparenchymal hemorrhages were traumatic. Most of the patients with ICH experienced some type of neurologic abnormality (159 [97.0%] patients), 3 (1.8%) patients presented with only headache, and 2 (1.2%) patients were asymptomatic (ICH found in regular computerized tomography after head trauma). Overall, 107 (61.5%) ICH events fulfilled criteria for hemorrhagic stroke: 91 (85.0%) intraparenchymal hemorrhages, 9 (8.4%) subdural hematomas, 5 (4.7%) subarachnoid hemorrhages, and 2 (1.9%) with unknown location.
Patient characteristics
Baseline characteristics according to the occurrence and location of ICH are presented in Table 2. Patients with ICH were older, weighed less, and had more history of prior embolic events than those without ICH. Additionally, patients with ICH were more likely to have renal dysfunction at baseline and use of aspirin at time of randomization. The frequency of prior clinically relevant or spontaneous bleeding was similar between those with and without ICH, as was prior use of a vitamin K antagonist in the 30 days before randomization. A trend toward a higher frequency of history of falls in the last year was observed among patients with ICH (7.6% vs 4.5%; P = .068) versus those without ICH. Blood pressure at admission with ICH was available for 81 (46.6%) patients: mean systolic blood pressure was 160 mm Hg and mean diastolic blood pressure was 90 mm Hg. The median systolic and diastolic blood pressures at the last study visit before the ICH event were 131 mm Hg (120, 142 mmHg) and 80 mm Hg (70, 87 mm Hg), respectively. These values were similar in patients receiving warfarin and apixaban. The median number of days from last blood pressure measurement to the ICH event was 41 (17, 64 days).
Antithrombotic drugs
Among patients with ICH, 70.1% were randomized to warfarin and 29.9% to apixaban. Among warfarin-treated patients with ICH, the median INR prior to ICH was 2.6 (2.1, 3.0); 78.5% had a pre-ICH INR within or below the therapeutic range (≤ 3.0) for the most recent measurement before ICH (median time from INR to event: 13 days). INR values were available for 8 patients the day before the ICH event: 5 values were between 2.0 and 3.0, 2 values were < 2.0, and 1 value was > 3.0. There were also 4 patients with INR values on the day of the ICH event: 3 values were between 2.0 and 3.0, and 1 value was > 3.0. Only 2 patients in the apixaban group received a reduced dose of apixaban (2.5 mg twice daily).
Of all patients with ICH, 66 (37.9%) were on aspirin at baseline, and half had a formal indication for aspirin, such as previous history of stroke/TIA, myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, or peripheral artery disease. Among those with ICH, 54 (31%) were on aspirin the day before the ICH, and 4 (2.3%) were on thienopyridines.
Treatment effects of apixaban versus warfarin by ICH type and location
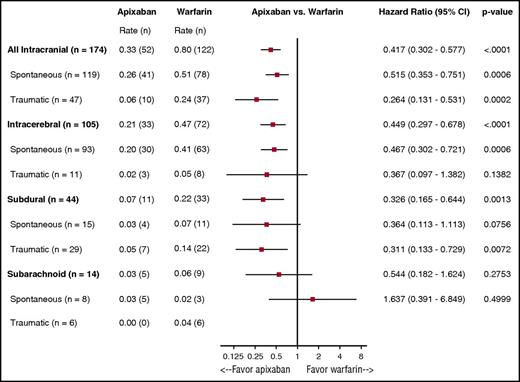
The rates of ICH by type, location, and randomized treatment are presented in Figure 1. Apixaban resulted in less ICH overall (HR: 0.42, 95% CI: 0.30-0.58; P < .0001), spontaneous ICH (HR: 0.52, 95% CI: 0.35-0.75; P = .0006), and traumatic ICH (HR: 0.26, 95% CI: 0.13-0.53; P = .0002). Patients treated with warfarin more often experienced intraparenchymal and subdural hemorrhages when compared with those receiving apixaban (0.47% per year vs 0.21% per year; 0.22% per year vs 0.07% per year [warfarin vs apixaban]). Similar results were seen for subarachnoid hemorrhages and traumatic intracerebral bleeding events.
Rates of ICH by location, type, and randomized treatment. The benefits of apixaban over warfarin in causing significantly less ICH was consistent regardless of type and location of ICH.
Rates of ICH by location, type, and randomized treatment. The benefits of apixaban over warfarin in causing significantly less ICH was consistent regardless of type and location of ICH.
Predictors of ICH
Independent predictors of ICH are described in Table 3. No interaction was seen between randomized treatment and region, prior stroke/TIA, or aspirin use at randomization. However, a randomized treatment-by-age quantitative interaction was seen (interaction: P = .04), indicating that apixaban had an even greater effect in causing less ICH in older versus younger patients. Using age as a continuous variable, the HR (95% CI) for ICH for apixaban versus warfarin in those 55 years of age was 0.78 (0.40-1.51), 0.53 (0.36-0.79) for those 65 years of age, and 0.37 (0.26-0.53) for those 75 years of age. The ICH event rates by ATRIA, HAS-BLED, and ORBIT bleeding scores are presented in Supplemental table 1.
Management of ICH
Among patients for whom the type of treatment was available (n = 161), surgical intervention was performed in 36 patients (22.4%). Of those, 21 had subdural hemorrhages, 12 had intraparenchymal bleeding, and 3 had subarachnoid hemorrhages.
Among those with ICH and available bleeding management information (n = 171), 23 (13.5%) were given vitamin K or other medications to stop the bleeding within 3 days of ICH (2 in the apixaban group, 21 in the warfarin group). Of those, 17 received vitamin K (1 in the apixaban group, 16 in the warfarin group); 4 received either 4-factor prothrombin complex concentrate, 3-factor prothrombin complex concentrate, or recombinant factor VIIa (1 in the apixaban group, 3 in the warfarin group); and 2 patients in the warfarin group received either etamsylat or carbozochrome sulfonate hydrate and menantetrenone. A total of 14 (8.2%) patients received blood transfusions within 3 days of ICH (2 in the apixaban group, 12 in the warfarin group). Fresh frozen plasma was given to 10 patients (2 in the apixaban group, 8 in the warfarin group). In the warfarin group, 2 patients received fresh frozen plasma and red blood cells; 1 received fresh frozen plasma and platelets; and 1 received fresh frozen plasma, red blood cells, and platelets.
Mortality after ICH
Kaplan-Meier mortality rates following ICH were 43.3% at 30 days, 45.3% at 90 days, and 47.6% at 6 months. The mortality rates per 100 patient-years of follow-up were 88.8% for the ICH group and 3.5% for the non-ICH group. These estimates are based on 81 deaths in 91.23 years of follow-up for patients in the ICH group and 1189 deaths in 33 952.43 years of follow-up for patients in the non-ICH group.
Of the 96 patients with nonfatal ICH, 94 (97.9%) were contacted at ≥30 days after the ICH. The modified Rankin scale at discharge was ≥ 4 in 55.7% of those with ICH (Supplemental table 2). Almost half of the survivors with follow-up records available experienced at least some neurologic deficit after the event (modified Rankin scale ≥2). Among survivors, disability was at least of a moderate degree in 37.8% of patients.
Among the 66 patients who died before discharge after ICH with ICH location information available, 47 (71.2%) had intraparenchymal hemorrhages, 15 (22.7%) had subdural hematomas, and 4 (6.1%) had subarachnoid hemorrhages. The 30-day Kaplan-Meier rates of death following ICH were 45.6% for intraparenchymal hemorrhages, 35.6% for subdural hematomas, and 21.4% for subarachnoid hemorrhages. Mortality rates following ICH at 30 days were 45.4% in the apixaban group and 42.6% in the warfarin group (Figure 2). Overall, 7 patients had ischemic stroke within 30 days after the ICH event (2 in the apixaban group, 5 in the warfarin group); all 7 patients were off the study drug at the time of the ischemic stroke.
Thirty-day all-cause mortality after ICH event by randomized treatment. Similar rates of all-cause mortality following ICH were observed in apixaban- and warfarin-treated patients.
Thirty-day all-cause mortality after ICH event by randomized treatment. Similar rates of all-cause mortality following ICH were observed in apixaban- and warfarin-treated patients.
Discussion
In patients with AF assigned to oral anticoagulation, ICH occurred at a rate of 0.80% per year with warfarin and 0.33% per year with apixaban, with significantly lower rates on apixaban regardless of ICH type (traumatic or spontaneous) or location (intraparenchymal, subdural, or subarachnoid). Importantly, nearly 80% of patients with ICH receiving warfarin had a pre-ICH INR within or below therapeutic range at an average of 2 weeks before the ICH event, suggesting that better control of INR might not necessarily have a significant impact on reducing ICH in the warfarin population. Concomitant aspirin use was independently associated with an increased risk of ICH. Of note, only half of the patients using aspirin had a formal indication for its use, such as previous stroke/TIA, coronary artery disease, or peripheral artery disease. It has been shown that adding aspirin in addition to oral anticoagulant therapy in patients with AF and stable coronary artery disease or history of stroke/TIA does not provide additional clinical benefit.13-15 This suggests that avoiding unnecessary aspirin could be a strategy to reduce ICH risk. Other risk factors associated with ICH were older age, prior stroke/TIA, and enrollment in Asia or Latin America. ICH was associated with a high 30-day mortality rate and at least moderate disability in approximately one-third of the survivors. Similar rates of all-cause mortality following ICH were observed in apixaban- and warfarin-treated patients.
Similar to our findings, other studies have shown crude annual rates of ICH around 1.0% in patients with AF treated with oral anticoagulants over a 1- to 2-year time frame. These reports also demonstrated high associated 30-day mortality rates following an ICH event.8-10 In the Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY) trial, 153 patients presented with ICH during follow-up (0.85%), 36% of whom died within 30 days.8 In the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF), 172 patients (1.2% of the overall population) experienced ICH, and the fatality rate was 43% within 30 days.9 In the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial, 234 (1.1%) patients presented with ICH; of those, 33.3% had fatal ICH.10 Even though ICH was uncommon in the recent NOAC trials, it was consistently associated with a high mortality rate, illustrating the importance of preventing this devastating complication.
Some well-established predictors of ICH were confirmed in our study, as well as in previous NOAC ICH analyses.7-9,16 Older age, previous stroke/TIA, and warfarin use were factors associated with increased risk of ICH in RE-LY, ROCKET AF, and our study. In addition, the use of aspirin was associated with ICH in RE-LY and in our study. Interestingly, a history of falls was not a significant predictor of ICH in any of the pivotal NOAC trials. The ability to identify modifiable factors associated with ICH in patients with AF receiving oral anticoagulant therapy is clinically important (such as avoiding aspirin and using a NOAC instead of warfarin), because information on such factors may help physicians provide the best clinical benefit for patients requiring anticoagulant therapy.
The concern and perception about the risk of trauma and falls as well as the lack of reversal agents for most of the NOACs have likely influenced health care providers to not prescribe an appropriate anticoagulant for stroke prevention in eligible patients with AF, particularly older patients with comorbidities. It has been shown that health care providers overestimate the risk of ICH by 10 times when compared with the actual risk.17 Although there is little good evidence that quantifies the degree to which “fall risk” or a “history of falls” increases the risk of serious ICH, the ICH rate per 100 patient-years among patients at a high risk for falls is 2.8%, whereas the ischemic stroke rate per 100 patient-years in the same population is 13.7%.18 Thus, the use of anticoagulant therapy in patients with AF does not appear to be mainly driven by perceived benefits. Conversely, perceived risks appear to strongly affect decision-making about anticoagulant therapy in clinical practice.
Until recently, the lack of specific reversal agents for NOACs raised concerns about their use. It is known that the effect of warfarin can be reversed with vitamin K or fresh frozen plasma; however, the extent to which using warfarin reversal strategies translate to favorable outcomes is yet to be confirmed.19 Despite that, there are studies that demonstrated that prothrombin complex concentrate administration reduces hematoma enlargement in patients with spontaneous ICH treated with vitamin K antagonists.20 Most importantly, in patients with supratherapeutical anticoagulation (a major risk factor for hematoma enlargement), vitamin K antagonist reversal may help prevent small hematomas from becoming life-threatening hemorrhages. The mortality rate of ∼50% after ICH is high, and there is a potential for optimized anticoagulation reversal strategies to improve patient outcomes.
One of the most favorable features of NOACs in relation to the risk of bleeding is their relatively short half-life. Their shorter half-life and lower rates of ICH explain, at least in part, the lower fatality rates after major bleeding on NOACs when compared with warfarin.11,21-25 Our findings illustrate that the lack of specific reversal agents for NOACs should not be a major consideration, and the best treatment of ICH events is to prevent them from occurring in the first place. Nevertheless, specific reversal agents for NOACs are being developed and approved.26-28 Recently, it was shown that andexanet alfa, a factor Xa antidote, reduced anti–factor Xa activity in patients with major bleeding while using factor Xa inhibitors.29 Thus, the availability of reversal agents of factor IIa and Xa inhibitors may have some role in special situations, such as severe and emergent bleeding events.
Importantly, dabigatran, rivaroxaban, apixaban, and edoxaban have significantly reduced the rates of ICH as compared with warfarin.10,11,22,23 In our analysis, a substantial reduction in the ICH rate was shown with apixaban regardless of ICH type (spontaneous or traumatic) and location (intraparenchymal, subdural, or subarachnoid), as compared with warfarin. The reduction in ICH with apixaban seems to be even greater in older patients. The mechanism behind this finding is unknown, and it may not be solely related to poor INR control or any brain-specific interaction because intraparenchymal, subdural, and subarachnoid hemorrhages have different pathophysiological mechanisms. In addition, we have shown that apixaban compared with warfarin causes less ICH, especially among those at high risk for bleeding as defined by a HAS-BLED score ≥3.30 Finally, almost 80% of warfarin-treated patients who experienced an ICH in ARISTOTLE had a pre-ICH INR lower than 3.0 two weeks before the event. In clinical practice, it is often difficult to maintain a well-controlled warfarin level, but even when this good control is achievable, our findings suggest that it may not completely prevent the occurrence of ICH. Thus, NOACs represent an evolutionary step in the care of patients with AF with, or at risk for, thromboembolic events, leading to signficantly less ICH compared with warfarin as well as less fatal bleeding.3,11,21-25,31
Our study has several limitations. As a secondary analysis of a clinical trial, non-randomized comparisons were performed and residual confounding is likely present, despite statistical adjustments. Additionally, we had information about INR values and blood pressure at the time of the ICH event only for a few patients. Therefore, these variables were not included in our prediction models. However, we had INR values on average 2 weeks before the ICH event and they were below 3.0 in approximately 80% of the patients with ICH. Finally, a small number of subarachnoid hemorrhages limited the analysis of treatment effect in this group.
In conclusion, in the ARISTOTLE trial, the rate of ICH in patients with AF assigned to oral anticoagulation was 0.80% per year with warfarin regardless of INR control and 0.33% per year with apixaban, and ICH was associated with high short-term morbidity and mortality. Our findings highlight the clinical relevance of reducing ICH by using apixaban rather than warfarin and avoiding concomitant aspirin, especially in patients with older age.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Editorial assistance was provided by Elizabeth Cook, an employee of the Duke Clinical Research Institute.
The ARISTOTLE study was funded by Bristol-Myers Squibb (Princeton, NJ) and Pfizer, Inc. (New York, NY) and coordinated by the Duke Clinical Research Institute and Uppsala Clinical Research Center (Uppsala, Sweden). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. In the main ARISTOTLE trial, there was 1 research representative from the sponsor on the steering committee. The lead author (R.D.L.) had full access to the data and all authors agreed to submit the results of the analysis for publication.
Authorship
Contribution: R.D.L., L.W., J.H.A., and C.B.G. contributed to the data collection and study design; R.D.L., P.O.G., B.J.K., D.M.W., C.D.B., M.H., J.D.E., L.T., L.W., S.M.A.-K., C.H., P.G.M.d.B.e.S., J.H.A., C.B.G., and H.-C.D. contributed to the data interpretation and writing of the manuscript; D.M.W. and L.T. performed the statistical analysis; and R.D.L., P.O.G., B.J.K., D.M.W., C.D.B., M.H., J.D.E., L.T., L.W., S.M.A.-K., C.H., P.G.M.d.B.e.S., J.H.A., C.B.G., and H.-C.D. had full access to the data.
Conflict-of-interest disclosure: R.D.L. received institutional research grants from Bristol-Myers Squibb and GlaxoSmithKline and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Merck, Pfizer, and Portola. J.H.A. received institutional research grants from Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Pfizer, Sanofi, Regado Biosciences, Tenax, and Vivus and consulting fees/honoraria from Bristol-Myers Squibb, CSL Behring, Daiichi Sankyo, GlaxoSmithKline, Janssen, Pfizer, Portola, Sohmalution, and Xoma. C.D.B. received research salary support from the Patient Centered Outcomes Research Institute and the American Heart Association. J.D.E. serves on advisory boards for AstraZeneca, Genetech, and Sanofi-Aventis; Data Monitoring Boards for Daiichi-Sankyo, Schering-Plough Research Institute, and Novartis and received consulting fees from Bristol-Myers Squibb. L.W. received an institutional research grant, consultancy and lecture fees, travel support, and honoraria from GlaxoSmithKline; institutional research grants, consultancy and lecture fees, and travel support from AstraZeneca, Bristol-Myers Squibb/Pfizer, and Boehringer Ingelheim; an institutional research grant from Merck & Co.; an institutional research grant from Roche; consultancy fees from Abbott; and holds 2 patents involving growth differentiation factor-15. C.H. has received research grants from GlaxoSmithKline, Merck, Roche, Bristol-Myers Squibb, AstraZeneca and speakers bureau fees from AstraZeneca. C.B.G. has received grants and personal fees from GlaxoSmithKline, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Sanofi-Aventis, Takeda, The Medicines Company, Janssen, and Bayer; grants from the Medtronic Foundation, Merck & Co., and Armetheon; and personal fees from Hoffmann-La Roche, Lilly, AstraZeneca, Daiichi Sankyo, Ross Medical Corporation, Salix Pharmaceuticals, Gilead, and Medtronic. H.-C.D. has received honoraria/advisory board fees from Abbott, Allergan, AstraZeneca, Bayer Vital, Bristol-Myers Squibb, Boehringer Ingelheim, CoAxia, Corimmun, Covidien, Daiichi-Sankyo, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, Johnson & Johnson, Knoll, Lilly, Merck Sharp & Dohme, Medtronic, MindFrame, Neurobiological Technologies, Novartis, Novo Nordisk, Paion, Parke-Davis, Pfizer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Syngis, Talecris, Thrombogenics, WebMD Global, Wyeth, and Yamanouchi and support for research projects from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Lundbeck, Novartis, Janssen-Cilag, Sanofi-Aventis, Syngis and Talecris. The remaining authors declare no competing financial interests.
Correspondence: Renato D. Lopes, Duke Clinical Research Institute, Room 0311, Terrace Level, 2400 Pratt St, Durham, NC 27705; e-mail: renato.lopes@duke.edu.