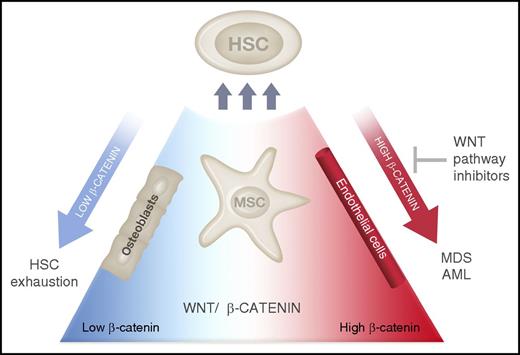
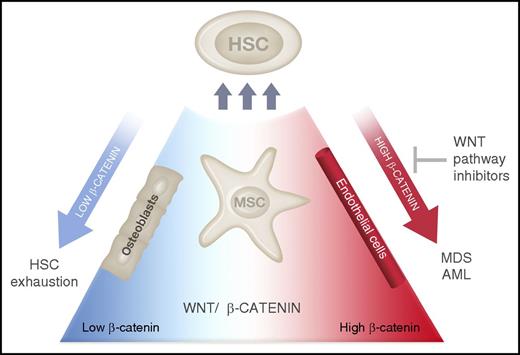
In this issue of Blood, Stoddart et al think outside of the stem cell “box” to identify a therapeutically tractable pathway in del(5q) myelodysplastic syndrome (MDS), through blocking pathologically activated Wnt signaling arising within the stem cell niche.1
HSC interactions with the niche are regulated by Wnt–β-catenin signaling. Expression of a Wnt antagonist from BM osteoblasts leads to HSC exhaustion,10 whereas activation of β-catenin within niche cells (MSCs and possibly osteoblasts or endothelial cells) induces the transformation to MDS or AML. Inhibitors of Wnt–β-catenin signaling may prevent the development of anemia and prolong survival in MDS. Illustration by Madeleine Flynn, QIMR Berghofer Medical Research Institute.
HSC interactions with the niche are regulated by Wnt–β-catenin signaling. Expression of a Wnt antagonist from BM osteoblasts leads to HSC exhaustion,10 whereas activation of β-catenin within niche cells (MSCs and possibly osteoblasts or endothelial cells) induces the transformation to MDS or AML. Inhibitors of Wnt–β-catenin signaling may prevent the development of anemia and prolong survival in MDS. Illustration by Madeleine Flynn, QIMR Berghofer Medical Research Institute.
The stem cell niche is a complex heterotypic microenvironment that instructs normal hematopoietic stem cell (HSC) development. Deregulation of this stem cell niche has been postulated to contribute to malignancy, leading to approaches to modulate niche signaling in both regenerative medicine and malignancy.2,3
Canonical Wnt signaling is an evolutionarily conserved developmental pathway that regulates self-renewal within benign and malignant stem cell populations. Adenomatous polyposis coli (APC) together with glycogen synthase kinase 3β (GSK-3β), AXIN, and casein kinase 1 form part of a complex that phosphorylates and inactivates β-catenin through cytoplasmic localization and proteasomal degradation. β-catenin (Ctnnb1) has myriad cellular functions including the transcriptional regulation of self-renewal, survival, and cell polarity. Pathological activation of β-catenin signaling has been demonstrated within leukemia stem cells (LSCs) in myeloid malignancies including chronic and acute myeloid leukemia (AML).4,5 In a subtype of MDS associated with partial deletion of the long arm of chromosome 5 (known as del(5q) MDS), APC is located on 5q within the common deleted region resulting in a haploinsufficient state.6,7 Murine models of Apc haploinsufficiency demonstrate MDS-like changes including a lethal macrocytic anemia and red cell developmental abnormalities.7,8 Interestingly, this model of MDS was regulated by factors extrinsic to the HSCs, providing a fascinating example of MDS driven by the stem cell niche. Recently, expression of a constitutively active form of β-catenin from the niche gave rise to AML associated with recurrent genetic abnormalities.9 This group also identified β-catenin activation within the niche in a significant proportion of patients with AML. Altogether, these findings suggest therapeutic targeting of Ctnnb1 may be an effective approach in myeloid malignancies.
Stoddart et al have now used a series of elegant genetic experiments to demonstrate activation of canonical Wnt–β-catenin signaling within bone marrow (BM) mesenchymal stem cells (MSCs) of Apc-haploinsufficent mice. Targeted deletion of 1 Ctnnb1 allele within MSCs prevented the development of MDS in the Apc+/− mice, delayed the onset of anemia, and prolonged survival in vivo. Detailed mechanistic analysis showed that this phenotypic rescue corresponded to reduced canonical Wnt signaling within MSCs, validating the specificity of this approach. The authors subsequently identified pyrvinium, a US Food and Drug Administration–approved antihelminthic drug, as a novel Wnt–β-catenin inhibitor. By administering pyrvinium to Apc-haploinsufficent mice, they were able to rescue the anemia and prolong survival, again correlating this in vivo mechanism with inhibition of β-catenin activation within MSCs. Finally, they validated the efficacy of pyrvinium to inhibit β-catenin activation in primary, patient-derived del(5q) MDS samples (see figure). In the current study, pyrvinium was able to prevent, or delay, the development of anemia, but was less effective in ameliorating established anemia. This suggests that early identification and treatment of susceptible patients is essential to the success of this approach.
Like many excellent studies, this work raises a number of ongoing questions. First, how frequently is β-catenin activated within the MDS niche, and is this activation linked to the MDS cytogenetic or molecular genotype? Does aberrant β-catenin signaling contribute to clonal development and AML progression from MDS,9 and is it required for maintenance of LSCs? Which are the pathologic cell populations within the MDS niche and what is the best compound to therapeutically target β-catenin activation in vivo? Finally, how do we identify at-risk MDS patients early enough in their disease that they are likely to benefit from this approach?
Clinical hematology is in the midst of a genomics revolution, with the increasingly widespread availability of next-generation sequencing for blood cancers. For practicing clinicians, the application of these data are often challenging, and approaches that focus on common, deregulated pathways offer practical and pragmatic solutions. With the current study, Stoddart and colleagues provide a novel approach to manipulate the stem cell niche, an avenue that is likely to have broad implications and may help treat patients with MDS and related malignancies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.