Key Points
Risk of HF increases following cardiac radiation doses above 20 Gy.
Anthracyclines increase HF rate by threefold independently of radiation.
Abstract
Hodgkin lymphoma (HL) survivors treated with radiotherapy and/or chemotherapy are known to have increased risks of heart failure (HF), but a radiation dose-response relationship has not previously been derived. A case-control study, nested in a cohort of 2617 five-year survivors of HL diagnosed before age 51 years during 1965 to 1995, was conducted. Cases (n = 91) had moderate or severe HF as their first cardiovascular diagnosis. Controls (n = 278) were matched to cases on age, sex, and HL diagnosis date. Treatment and follow-up information were abstracted from medical records. Mean heart doses and mean left ventricular doses (MLVD) were estimated by reconstruction of individual treatments on representative computed tomography datasets. Average MLVD was 16.7 Gy for cases and 13.8 Gy for controls (Pdifference = .003). HF rate increased with MLVD: relative to 0 Gy, HF rates following MVLD of 1-15, 16-20, 21-25, and ≥26 Gy were 1.27, 1.65, 3.84, and 4.39, respectively (Ptrend < .001). Anthracycline-containing chemotherapy increased HF rate by a factor of 2.83 (95% CI: 1.43-5.59), and there was no significant interaction with MLVD (Pinteraction = .09). Twenty-five–year cumulative risks of HF following MLVDs of 0-15 Gy, 16-20 Gy, and ≥21 Gy were 4.4%, 6.2%, and 13.3%, respectively, in patients treated without anthracycline-containing chemotherapy, and 11.2%, 15.9%, and 32.9%, respectively, in patients treated with anthracyclines. We have derived quantitative estimates of HF risk in patients treated for HL following radiotherapy with or without anthracycline-containing chemotherapy. Our results enable estimation of HF risk for patients before treatment, during radiotherapy planning, and during follow-up.
Medscape Continuing Medical Education online
This activity has been planned and implemented through the joint providership of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the American Nurses Credentialing Center (ANCC), the Accreditation Council for Pharmacy Education (ACPE), and the Accreditation Council for Continuing Medical Education (ACCME), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2334.
Disclosures
Associate Editor Laurie H. Sehn served as an advisor or consultant for AbbVie, Amgen, Roche/Genentech, Celgene, Janssen, Lundbeck, Seattle Genetics, and TG Therapeutics. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Alnylam, Biogen, and Pfizer. The authors declare no competing financial interests.
Learning objectives
Distinguish the risk for heart failure (HF) in patients with Hodgkin lymphoma (HL) related to radiation exposure vs no exposure, based on a case-control study.
Distinguish the risk for HF in patients with HL related to anthracycline exposure.
Determine the clinical implications regarding the risk for HF in patients with HL related to radiation and anthracycline exposure.
Release date: April 20, 2017; Expiration date: April 20, 2018
Introduction
With 10-year survival rates >80%, Hodgkin lymphoma (HL) is a model of a curable malignancy.1 The efficacy and safety of treatments continue to improve, but late effects, including cardiovascular diseases, have caused substantial treatment-related morbidity and mortality in HL survivors treated in the past. It has been shown that mediastinal radiotherapy and/or anthracycline-containing chemotherapy increase the risk of coronary heart disease (CHD), valvular heart disease (VHD), and heart failure (HF) in HL survivors.2-9
We previously found a linear dose-response relationship for the risk of radiation-related CHD in HL survivors with the excess rate increasing by 7.4% per Gy,10 and a nonlinear dose-response relationship for VHD, with 1.4-, 3.1-, 5.4-, and 11.8-fold increased VHD rates for doses of ≤30 Gy, 31-35 Gy, 36-40 Gy, and >40 Gy, respectively.3 Mediastinal radiotherapy has also been associated with HF in survivors of both HL and childhood cancer.9,11 However, the shape of the radiation dose-response relationship has not previously been described, either for patients who received radiotherapy alone or for patients who received radiotherapy in combination with anthracyclines. Radiotherapy use has declined but still remains an important component of HL treatment. Anthracyclines have been commonly used since the early 1980s and remain the backbone of HL chemotherapy regimens used today.
With this study, we aimed to determine the radiation dose-response relationship for HF in long-term survivors of adolescent and adult HL, to estimate cumulative risk of HF after radiotherapy given with and without anthracyclines, and to assess other determinants of HF risk.
Methods
Study population
A nested case-control study was conducted within an existing multicenter hospital-based cohort (N = 2617) of HL survivors treated in The Netherlands between 1965 and 1995 before the age of 51 years, who had survived ≥5 years after HL diagnosis. The cohort was identified through hospital-based cancer registries in 4 large university hospitals and 1 cancer center. Over time, a wide variety of treatments was used in the cohort. The majority of patients were treated according to European Organization for Research and Treatment of Cancer Lymphoma Group protocols for primary treatment.12 Briefly, in the 1960s, patients were treated with orthovoltage therapy or cobalt-60; from the 1970s onwards, linear accelerators were used. Individual blocks were used to shield normal tissues as much as possible. Patients usually received 40 Gy in fractions of 1.5 to 2.0 Gy when they were treated with radiotherapy only and 30 to 36 Gy when they also received chemotherapy. Mantle field irradiation (including mediastinal, axillary, and neck nodes) was the most commonly applied radiation from the early 1970s to the late 1980s. Since the late 1980s, a growing number of patients received more limited radiation fields (involved fields). Treatment of recurrences was generally less standardized.
From the 1960s to 1980s, chemotherapy consisted mainly of MOPP (mechlorethamine, vincristine, procarbazine, prednisone). In the 1980s, anthracycline-containing regimens such as MOPP/ABV (MOPP/doxorubicin, bleomycin, vinblastine) and ABVD (ABV and dacarbazine) were introduced as part of primary treatment. Anthracycline dose was estimated in milligrams anthracycline per square meter body surface, based on number of cycles received times the standard anthracycline dose in the corresponding chemotherapy regimen during that time period. Standard doses of anthracycline per regimen per cycle were 25 mg/m2 at days 1 and 15 for ABVD and alternating MOPP-ABVD and 35 mg/m2 at day 8 for MOPP-ABV hybrid.
A detailed description of patient selection, data collection, and treatments has been published previously2,9,13-15 as well as assessment of cardiovascular events during follow-up.9 Patients were eligible for this study if (1) they survived at least 5 years after HL diagnosis; (2) they were diagnosed with HL before the age of 51 years; (3) HL was their first primary malignancy (except for nonmelanoma skin cancer or carcinoma in situ of the cervix uteri or the breast); and (4) radiotherapy for HL was the only radiotherapy given to the neck or trunk prior to the cutoff date, which was defined as date of HF for the cases, whereas for each control it was defined as date of HL diagnosis plus a time interval equal to the interval from date of HL diagnosis to date of HF diagnosis of the corresponding case.
Cases and controls
Cases (n = 91) were patients who developed HF in the form of either symptomatic congestive HF or cardiomyopathy, with an ejection fraction of <50% (or a ≥10% drop from baseline) or a fractional shortening of <24% (based on a combination of the Common Terminology Criteria for Adverse Events versions 3.0 and 4.0: grade ≥2; see supplemental Text 1, available on the Blood Web site)16 as their first clinically significant heart disease. Cases were identified from medical records or postal questionnaires completed by the patient’s general practitioner (GP)9 and verified using cardiac notes from either the GP or the treating cardiologist. The data abstraction forms and coding instructions were developed in collaboration with physicians, and it has been shown that the Common Terminology Criteria for Adverse Events can be used to properly grade cardiovascular events from medical records.17 A total of 53 further cases of HF were excluded (see supplemental Table 1 for reasons). Follow-up was complete to October 2013. For each case with HF, we attempted to select 4 controls from the cohort, individually matched on sex, age at HL diagnosis (≤1 year), and date of HL diagnosis (≤3 years). Controls had to be free of any cardiac disease grade ≥2 at the cutoff date. In total, 278 controls were matched to the cases. Cases were eligible to be controls up to the date they developed HF, and controls were selected with replacement.
Data collection
Detailed treatment information, including radiation doses and fields and cumulative chemotherapy doses, was collected from medical records. Copies of original radiotherapy prescription sheets and simulation films were obtained. Where original prescriptions were unavailable, information about radiotherapy, including dates, anatomical areas, dose, fractionation, and treatment energy, was abstracted from other clinical notes. If cumulative chemotherapy doses were not available, regimen-specific standard doses were multiplied by the number of cycles that a patient received. Cardiotoxicity equivalence ratios of 0.50 for daunorubicin and epirubicin to doxorubicin were used.18,19 Vital status and dates of death were obtained up to July 2013 by linkage with the Dutch Central Bureau of Genealogy. In The Netherlands, the law requires that GP and hospital records must be kept throughout a patient’s lifetime and for at least 15 years after their death. Detailed data on medical history, smoking, and established cardiovascular risk factors, both at diagnosis of HL and at diagnosis of HF (or cutoff date for controls), could therefore be collected for all patients from GP questionnaires in 2004 (for 94% of the cohort) and in 2013 (for 83% of the cohort), and from hospital records. In addition, a questionnaire on established cardiovascular risk factors and lifestyle was mailed to all patients in the cohort still alive in 2013 (n = 475; response rate: 70%), resulting in questionnaire data for 45 cases and 186 controls. Further details are given elsewhere.2,10,20 This study was approved by the ethics review board of The Netherlands Cancer Institute.
Retrospective radiation dosimetry methods
The radiation dosimetry method is described elsewhere.3 Radiotherapy regimens were reconstructed using the Eclipse treatment planning system (Version 13.0.28; Varian Medical System, Palo Alto, CA). Two substitute computed tomography (CT) data sets (for men and women, respectively) were chosen from a library of 50 to be representative regarding average anatomy and estimated heart dose from a standardized mantle field. The heart and substructures of the heart were outlined as per published guidelines.21 Treatment planning was performed for each individual patient using variables such as beam arrangement, energy, prescribed dose, field size, and field shielding, which were extracted from each patient’s original radiotherapy prescription charts and simulation films. Standard mantle fields, as well as paraaortic and splenic fields for patients who received such treatments, were created. The dose distributions from all fields were then summed, and cardiac doses were extracted. Mean heart dose (MHD) and mean left ventricular dose (MLVD) were calculated and converted into equivalent dose in 2-Gy fractions (EQD2) and biologically effective dose.22,23 When fraction size varied during treatment, EQD2 and biologically effective dose were calculated separately for each fraction size before summation (supplemental Text 2). V20 and V30 (volume of structure receiving at least 20 or 30 Gy, respectively) were calculated for the heart and left ventricle and expressed in percentages.
For patients with neither radiation chart nor simulation film (22 cases and 103 controls), MHD and MLVD were estimated, for each combination of hospital, treatment period, sex, and radiation field, as the average value for patients with either a chart or a film.
Statistical analysis
Rate ratios (RRs) for HF for different levels of each factor were estimated using logistic regression, conditional on sets of individual cases and their matched controls. Confidence intervals (CIs) for factors with 2 levels were based on the Wald method. CIs for factors with >2 levels used the amount of information in each category, including the reference category.24 Multiple regression was used to control for confounding and to assess the combined effect of radiation dose and other factors. The dose-response was estimated by modeling HF rate as Κm(1 + βd), where d is radiation dose, Κm is a constant specific to each matched set, and β is the increase in excess HF relative rate per unit increase in dose. Nonlinearity was evaluated by including an exponential term: Κm[1 + βd·exp(δd)], and goodness of fit was assessed by likelihood ratio tests. Approximate cumulative risks of HF for categories of radiation dose were estimated from the HF RRs together with the cumulative risk of HF for the entire cohort (supplemental Text 3). Significance tests were 2-sided, and P ≤ .05 was taken to indicate statistical significance. Analyses were performed using STATA statistical software version 13.0 (STATAcorp 2013)25 and Epicure version 1.8 (Hirosoft International).26
Results
In the 91 cases, HF occurred after a median interval of 20.6 years (interquartile range [IQR]: 13.7-25.2) (Table 1). The majority of HF diagnoses were grade 2 (44%) or 3 (43%) (supplemental Table 1). The median age at HL diagnosis was 28.3 years (IQR: 21.9-37.7). Fifty-seven percent of the HF cases had died by the end of follow-up, with median time from HF to death of 3.6 years (IQR: 0.2-5.6).
Radiotherapy, chemotherapy, and splenectomy
For patients given mediastinal radiotherapy (cases: 90.1%; controls: 82.3%; Pdifference = .078), the average prescribed dose was 30.5 Gy (cases: 32.7 Gy; controls: 29.8 Gy; Pdifference = .088), whereas the average MHD was lower at 20.9 Gy (cases: 23.2 Gy; controls: 20.1 Gy; Pdifference = .009), and the average MLVD was even lower, at 14.5 Gy (cases: 16.7 Gy; controls: 13.8 Gy; Pdifference = .003). MHD and MLVD were strongly correlated (correlation coefficient: 0.93; supplemental Figure 1).
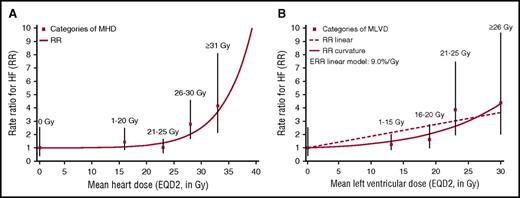
For all 3 measures of dose, HF rate increased with increasing dose (prescribed mediastinal dose: Ptrend = .027; MHD: Ptrend = .002; MVLD: Ptrend < .001; Table 2). For MHD, the dose-response relationship was nonlinear (Pcurvature = .029; supplemental Table 2), with little evidence of an increase for MHDs in the range 1 to 25 Gy, but increasing steeply with MHDs of ≥25 Gy (Figure 1A). For MLVD, there was no significant departure from linearity (Pcurvature = .09) (Figure 1B). HF rate also increased with increasing V30 and V20 for the left ventricle (Table 2). When the analysis was repeated omitting patients with neither radiation chart nor simulation film, results were similar (supplemental Table 3; supplemental Figure 2).
Relationship between HF rate and cardiac dose. RRs for HF by MHD (A) and by MLVD (B) in Gy compared with no radiation exposure. RRs are calculated conditionally on matched sets after adjustment for anthracycline-based chemotherapy (yes/no). Squares indicate anthracycline-adjusted estimates for the following dose categories: MHD: 0 Gy, 1-20 Gy, 20-25 Gy, 26-30 Gy, ≥31 Gy; MLVD: 0 Gy, 1-15 Gy, 16-20 Gy, 21-25 Gy, ≥26 Gy, and are plotted at the median dose in each category (0 Gy, 16 Gy, 23 Gy, 28 Gy, and 33 Gy for MHD; 0 Gy, 13 Gy, 19 Gy, 23 Gy, and 30 Gy for MLVD). Vertical lines are 95% CIs. For MHD, there was a statistically significant linear dose-response relationship (P = .006) and allowing for curvature improved the fit significantly (P ≤ .001). For MLVD, there was a statistically significant linear dose-response relationship (P = .004), and allowing for curvature did not significantly improve the fit (P = .09). Further details are given in supplemental Table 2.
Relationship between HF rate and cardiac dose. RRs for HF by MHD (A) and by MLVD (B) in Gy compared with no radiation exposure. RRs are calculated conditionally on matched sets after adjustment for anthracycline-based chemotherapy (yes/no). Squares indicate anthracycline-adjusted estimates for the following dose categories: MHD: 0 Gy, 1-20 Gy, 20-25 Gy, 26-30 Gy, ≥31 Gy; MLVD: 0 Gy, 1-15 Gy, 16-20 Gy, 21-25 Gy, ≥26 Gy, and are plotted at the median dose in each category (0 Gy, 16 Gy, 23 Gy, 28 Gy, and 33 Gy for MHD; 0 Gy, 13 Gy, 19 Gy, 23 Gy, and 30 Gy for MLVD). Vertical lines are 95% CIs. For MHD, there was a statistically significant linear dose-response relationship (P = .006) and allowing for curvature improved the fit significantly (P ≤ .001). For MLVD, there was a statistically significant linear dose-response relationship (P = .004), and allowing for curvature did not significantly improve the fit (P = .09). Further details are given in supplemental Table 2.
Chemotherapy without anthracyclines was not significantly associated with HF (RR: 0.93; 95% CI: 0.63-1.37). However, for anthracycline-based chemotherapy, the HF rate was increased by a factor of nearly 3 (RR: 2.83; 95% CI: 1.43-5.59). Among those who received anthracyclines, HF rates were similar for those with cumulative doses <280 mg/m2 and those with cumulative doses ≥280 mg/m2 (Pdifference = .97). HF rates were similar among those with and without splenectomy (RR: 0.85; 95% CI: 0.49-1.45). Among those who received mediastinal RT as primary therapy, HF rates did not differ significantly between those who received anthracyclines only as primary therapy and those who received anthracyclines only as salvage therapy (RR: 2.30; 95% CI 1.02-5.21 versus RR: 3.85; 95% CI 1.59-9.36; Pdifference = .4) (Table 2). Only 2 patients received anthracyclines for primary as well as salvage treatment; both were cases.
HF RRs in individuals with MHD or MLVD ≥26 Gy relative to 0 to 25 Gy did not differ significantly according to use of anthracycline chemotherapy (Pinteraction = .45 for MHD, .09 for MLVD) or with splenectomy (Pinteraction = .71 for MHD, .62 for MLVD).
Classical cardiovascular disease risk factors
None of the known classical cardiovascular disease risk factors differed significantly between HF cases and matched controls. Diagnosis of diabetes mellitus between HL and cutoff was associated with a nonsignificantly increased HF rate (RR: 1.59; 95% CI 0.63-4.05) compared with those not diagnosed with the disease (supplemental Table 4). When taking into account all risk factors that were diagnosed before the end of follow-up, instead of only those diagnosed prior to HF/cutoff date, hypertension (RR: 1.80; 95% CI: 0.73-4.44), diabetes mellitus (RR: 1.83; 95% CI: 0.96-3.47), and having at least 1 risk factor (RR: 1.53; 95% CI: 0.93-2.52) were all associated with nonsignificantly increased HF rates, whereas patients with a high level of physical activity (≥3 hours per week) at time of follow-up had a nonsignificantly lower HF rate than those who were not (<1 hour per week) (RR: 0.59; 95% CI: 0.32-1.10).
HF RRs in individuals with MHDs or MLVDs ≥26 Gy relative to 0-25 Gy did not differ significantly according to presence of at least 1 cardiovascular risk factor, sex, age at HL diagnosis, or time since HL diagnosis (all Pinteraction values >.50; supplemental Table 5A-B).
Estimated HF rates and cumulative risks
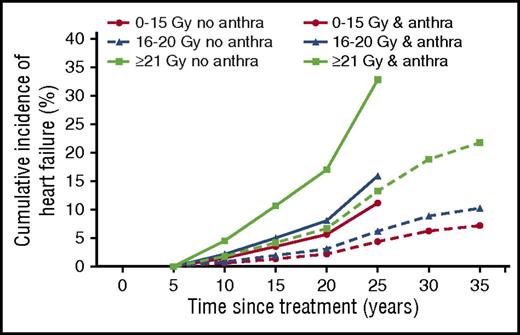
Our analyses showed that the only factors significantly associated with HF rate were radiation dose and whether anthracyclines were used, with no significant multiplicative interaction between the 2. Summary HF rates were therefore estimated based on 3 broad categories of MHD or MLVD and whether anthracyclines had been given, with the assumption that the multiplicative increase in HF rate with anthracyclines did not differ according to MHD or MLVD (Table 3). Based on these estimates, approximate cumulative incidence curves for HF were derived for patients in these 6 groups (Figure 2). In patients treated without anthracyclines, 25-year cumulative risks of HF following MLVDs of 0-15 Gy, 16-20 Gy, and ≥21 Gy were 4.4%, 6.2%, and 13.3%, whereas in patients treated with anthracyclines, the 25-year cumulative risks were 11.2%, 15.9%, and 32.9%, respectively. For patients treated without anthracyclines, 35-year cumulative risks of HF following MLVDs of 0-15 Gy, 16-20 Gy, and ≥21 Gy were 7.2%, 10.2%, and 21.8%, respectively. Patients treated with anthracyclines have not yet been followed long enough to estimate risks beyond 25 years.
Approximate cumulative risks of HF by MLVD and whether treatment with anthracyclines was given. Modeled cumulative risk of HF as first cardiac event among 5-year survivors of HL by time since initial HL treatment of categories of MLVD (Gy). Lines indicate estimated cumulative incidences for dose categories (0-15 Gy, 16-20 Gy, and ≥21 Gy) with and without anthracycline exposure. Cumulative risks were calculated with other heart disease or death as a competing risk. Further details are given in supplemental Text 3.
Approximate cumulative risks of HF by MLVD and whether treatment with anthracyclines was given. Modeled cumulative risk of HF as first cardiac event among 5-year survivors of HL by time since initial HL treatment of categories of MLVD (Gy). Lines indicate estimated cumulative incidences for dose categories (0-15 Gy, 16-20 Gy, and ≥21 Gy) with and without anthracycline exposure. Cumulative risks were calculated with other heart disease or death as a competing risk. Further details are given in supplemental Text 3.
Discussion
In this study, we have examined, for the first time, dose-response relationships for HF rate based on cardiac radiation exposure in 5-year survivors of adolescent or adult HL. We conducted analyses based on estimates of both MHD and MLVD derived from individual radiotherapy plans. Both measures of dose suggested that there is little increase in HF risk for lower doses, up to 25 Gy MHD or up to 15 Gy MLVD, but that HF rates increase rapidly at higher doses. We also found that treatment with anthracyclines increase the HF rate, approximately threefold, irrespective of cardiac radiation exposure.
A radiation dose-response relationship for self-reported HF has previously been observed in the Childhood Cancer Survivor Study (CCSS).11,27 In that cohort, HF risk was increased by factors of 1.6, 3.1, and 10.5 following MHDs of 5 to 14, 15 to 34, and ≥35 Gy, respectively, compared with patients who did not have any cardiac radiation exposure.27 These proportional increases are somewhat higher than those in our present study, possibly due to the patients' younger ages at cancer treatment: 82.4% of the CCSS cohort were aged <15 years at cancer diagnosis compared with only 3.5% in the present study. A clearly increasing HF risk with increasing anthracycline dose was also seen in the CCSS study, and risk was increased by factors of 2.1, 3.7, and 10.5 for cumulative anthracycline doses of <100, 100 to 249, and ≥250 mg/m2 compared with those not exposed to anthracyclines.
In the CCSS, the therapy-associated risk of HF was potentiated by the presence of cardiovascular risk factors, such as hypertension and diabetes mellitus, and by lack of physical activity.28,29 In our current study, no such associations were apparent. However, the CCSS studies were based on patient-reported outcomes of cardiovascular risk factors, and probably also included risk factors diagnosed at the same time as or even after the cardiovascular disease of interest, possibly resulting in an overestimation of the strength of the associations. In contrast, our conservative approach of including only risk factors diagnosed prior to HF may have resulted in an underestimation. Despite this, it seems likely that risk factor control in high-risk patients who received cardiotoxic treatment may be important in risk-reduction strategies for cardiovascular diseases after HL treatment.
The strengths of our study include that it was performed in a complete, multi-institutional population with long-term, detailed follow-up, including information from cardiologists and GPs. The outcome (HF) was GP reported and, if necessary, confirmed by cardiologists, which is an important advantage in comparison with studies relying on patient-reported outcomes or registry data.6,30-32 Instead of using prescribed radiation dose, radiation-related HF risks were estimated using individual patient dosimetric parameters such as MHD and MLVD, which were converted into EQD2 and adjusted for dose distribution and varying fractionation schedules. Where available, individual cumulative anthracycline doses were analyzed, rather than protocol prescription chemotherapy doses.
A limitation of our study is that a total of only 90 patients were prescribed anthracyclines, all but 11 of whom also received mediastinal radiotherapy. Furthermore, the range of cumulative anthracycline doses was limited, with most patients being prescribed either 280 or 300 mg/m2 doxorubicin equivalent, and follow-up for patients who received anthracyclines was shorter than for patients treated with radiotherapy alone. Therefore, our ability to study the interaction between radiation exposure of the heart and anthracycline exposure was limited, and we were unable to estimate a separate dose-response relationship for anthracyclines.
The results indicate that salvage therapy with anthracyclines following primary treatment with mediastinal radiotherapy would be more harmful than primary treatment with mediastinal radiotherapy and anthracyclines. However, the small number of patients treated with anthracyclines makes it impossible to draw any firm conclusions regarding the possible effect of the time elapsed between exposure of the heart to anthracyclines and to radiation. It is hypothesized that this increased risk is associated with an increased radiation dose during primary treatment, and perhaps an increased anthracycline dose for salvage treatment, compared with the doses for combined primary treatment. Further research may indicate whether treatment sequence may influence HF risk, which may contribute to risk prediction models of cardiovascular disease in survivors who have been treated in the past.
Detailed radiotherapy information was collected for each patient, but radiotherapy was applied before the era of CT-based treatment planning. Dose reconstructions were therefore carried out using representative CT datasets, and these cannot take into account patient-specific variations in heart size, shape, and position. For a proportion of patients, both the radiotherapy chart and the simulation film were missing, and the MHD and MLVD were estimated rather than reconstructed (see “Methods”). However, a sensitivity analysis including only patients for whom MHD and MLVD were reconstructed showed similar results (supplemental Table 3; supplemental Figure 2). Our study includes only cases in which HF was the first cardiovascular diagnosis, in order to eliminate the effects of other cardiovascular diseases such as CHD or VHD on the risk of developing HF. However, in a previous study,9 we showed that HF occurred frequently subsequent to CHD or VHD, rather than as a first event. Additional research is needed to study the influence of previous cardiovascular diseases and to identify risk factors for developing multiple cardiovascular diseases.
For patients treated today, MHDs and MLVDs will usually be well below the average values of 20.9 Gy and 14.5 Gy reported in this study, due to reductions in both treatment volumes and prescribed doses. Involved field radiotherapy has been reported to give a median MHD of 17.2 Gy for prescribed doses of 35 Gy and involved node radiotherapy leads to even lower doses (median MHDs of 7.7-12.0 Gy for prescribed doses of 36 Gy).33-35 Also, anthracycline doses are nowadays frequently lower than the doses received by the majority of patients in our study. Therefore, patients treated today are likely to be at a substantially lower risk of treatment-related HF than the patients included in this study. Studies in larger populations of HL survivors treated more recently would be helpful in characterizing the risks from modern treatments more precisely. More accurate dosimetry based on a patient’s individual CT scan may also help to determine whether MHD, MLVD, or another dosimetric parameter is the best measure to predict radiation-related HF risk. However, in the absence of more precise measures, these dose-response relationships for MHD and MLVD can be used to estimate HF risk both in patients treated today and in survivors treated in more recent decades.
In conclusion, cardiac radiation exposure and treatment with anthracyclines are important risk factors for the development of HF in HL survivors. Our findings are important for clinicians to provide information regarding risks of HF before treatment, during radiation treatment planning, and during long-term follow-up of HL survivors, including patients treated in the past.
Presented in abstract form at the Italian Association of Cardio-Oncology International Workshop on Cardio-Oncology, Naples, Italy, 1-3 October 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are very grateful to all patients who were willing to participate in this study. They would like to thank Karen Kooijman, Saskia Pelders, Merian van Wouwe, Sandra Fase, Kim Kersten, and Jannemieke Smit for their contributions to the data collection. They would also like to thank Frank Van Den Heuvel for his valuable advice as well as Yaochen Wang and Dan Warren for their assistance with the dosimetry analysis.
This study was supported by the Dutch Cancer Society (grant NKI 2010-4720) and by Cancer Research UK (grant C8225/A21133) as well as core funding from Cancer Research UK, the UK Medical Research Council, and the British Heart Foundation (BHF) to the Oxford University Clinical Trial Service Unit. This work was supported by the BHF Centre for Research Excellence at the University of Oxford (RE/08/04) (D.J.C.) and by the Nuffield Department of Population Health, University of Oxford (G.N.).
Authorship
Contribution: F.A.v.N., G.N., S.C.D., M.S., F.E.v.L., D.J.C., and B.M.P.A. designed the study and concept; F.E.v.L., S.C.D., G.N., and D.J.C. provided financial support; P.J.L., C.P.M.J., L.D., and B.M.P.A. provided study materials or patients; F.A.v.N., G.N., P.J.L., C.P.M.J., and L.D. collected and assembled the data: G.N. and D.J.C. conducted the dosimetry; F.A.v.N., G.N., S.C.D., M.S., M.H., F.E.v.L., D.J.C., and B.M.P.A. analyzed and interpreted the data; F.A.v.N., G.N., S.C.D., M.S., M.H., P.J.L., C.P.M.J., L.D., F.E.v.L., D.J.C., and B.M.P.A. wrote the manuscript; and F.A.v.N., G.N., S.C.D., M.S., M.H., P.J.L., C.P.M.J., L.D., F.E.v.L., D.J.C., and B.M.P.A. gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Berthe M. P. Aleman, Department of Radiation Oncology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: b.aleman@nki.nl.
References
Author notes
F.A.v.N. and G.N. contributed equally to this study.
D.J.C. and B.M.P.A. contributed equally to this study.