Key Points
Joint and bone healing abnormalities are demonstrated in hemophilic mice that are not solely because of the amount of blood in their joints.
Following initial hemostasis, extended FIX activity is needed in the week after hemarthrosis to normalize osteochondral healing.
Abstract
Wound healing requires interactions between coagulation, inflammation, angiogenesis, cellular migration, and proliferation. Healing in dermal wounds of hemophilia B mice is delayed when compared with hemostatically normal wild-type (WT) mice, with abnormal persistence of iron deposition, inflammation, and neovascularity. We observed healing following induced joint hemorrhage in WT and factor IX (FIX) knockout (FIX−/−) mice, examining also parameters previously studied in an excisional skin wound model. Hemostatically normal mice tolerated this joint bleeding challenge, cleared blood from the joint, and healed with minimal pathology, even if additional autologous blood was injected intra-articularly at the time of wounding. Following hemarthrosis, joint wound healing in hemophilia B mice was impaired and demonstrated similar abnormal histologic features as previously described in hemophilic dermal wounds. Therefore, studies of pathophysiology and therapy of hemophilic joint bleeding performed in hemostatically normal animals are not likely to accurately reflect the healing defect of hemophilia. We additionally explored the hypothesis that the use of a FIX replacement protein with extended circulating FIX activity could improve synovial and osteochondral wound healing in hemophilic mice, when compared with treatment with unmodified recombinant FIX (rFIX) in the established joint bleeding model. Significantly improved synovial wound healing and preservation of normal osteochondral architecture are achieved by extending FIX activity after hemarthrosis using glycoPEGylated FIX when compared with an equivalent dose of rFIX. These results suggest that treating joint bleeding only until hemostasis is achieved may not result in optimal joint healing, which is improved by extending factor activity.
Introduction
In the clinical care of hemophilia, the most common sites of bleeding are musculoskeletal. Recurrent joint bleeding leads to hemophilic arthropathy, which is the major morbidity of hemophilia. The pathogenesis of bleeding-induced joint degeneration in the past has been studied by exposing the synovium and cartilage of hemostatically normal animals to blood or blood components, either in vivo or ex vivo (eg, tissue explants).1-3 However, it is possible that studying healing of joint wounds in animals with intact hemostatic responses does not fully recapitulate healing processes in animals with impaired hemostatic potential.4-6 For this reason, we have recently modeled end points related to hemophilic intra-articular (IA) bleeding in hemophilia A and B mouse models. Specifically, a model of hemophilic gross joint hemorrhage induced by the puncture of the knee joint capsule with a fine gauge needle has been used by multiple research groups.7-15 This injury model is useful because hemostatically normal mice display minimal or no pathologic changes after a single puncture of the joint capsule, but hemophilic mice develop pathologic joint changes including synovial proliferation, exuberant neoangiogenic invasion, and blood and hemosiderin iron staining. These changes mirror human bleeding-induced arthropathy.7,8
During the clinical management of acute hemophilic joint hemorrhage, clotting factor is typically replaced IV until there is evidence that ongoing bleeding has stopped (eg, decreasing pain, stabilized joint swelling, improving joint range of motion). There is variance in clinical practice how long factor replacement is continued, if at all, after initial hemostasis is achieved.16,17
The current study explored the hypothesis that abnormalities will be observed in the time course and histopathology of healing in the blood-exposed joint in hemophilia B mice (when compared with mice with normal hemostasis). We hypothesized in addition that treating the hemarthrosis with a dose of clotting factor that corrects hemostasis will not restore normal joint wound healing, which will require instead prolonged restoration of thrombin generating potential.
Nonacog β pegol (N9-GP) is a recombinant factor IX (rFIX) protein that has been modified by attaching a single 40-kDa polyethylene glycol (PEG) molecule in an effort to prolong the circulating half-life of FIX zymogen. The half-life prolongation of N9-GP has been demonstrated in human prelicensure trials and has been associated with clinical resolution of target joint bleeding when infused as once-weekly prophylaxis.18-21 In addition, the pharmacokinetic profile of N9-GP in FIX−/− mice has been well characterized. N9-GP has a circulating half-life of 41 hours in this strain, compared with a 17-hour half-life for unmodified rFIX (Benefix).22 We explored the hypothesis that the use of a FIX protein that provides extended thrombin-generating potential (N9-GP) would achieve improved synovial and osteochondral wound healing in hemophilic mice when compared with treatment with unmodified rFIX.
Methods
Animal care and study
Wild-type (WT) C57Bl/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). FIX knockout C57Bl/6J (FIX −/−) male mice23 were bred in-house, were backcrossed to Jackson Laboratory C57Bl/6J for 12 generations (12N), and were age 7 to 10 weeks at the time of investigations. All investigations were approved by the University of North Carolina, Chapel Hill Institutional Animal Care and Use Committee. Mice were anesthetized using isoflurane/O2 for all procedures. All blood samples were collected from the retro-orbital plexus into 1:9 parts 3.2% citrated sodium and the platelet-poor plasma was stored at −80°C. The knee joint IA bleeding challenge results from the puncture of the joint capsule using a Hamilton syringe with a 30.5-G needle via a small (∼0.5 mm) incision of the skin overlying the patella as described.7-10 Following injury, all of the mice had access to Tylenol gel for pain relief. Knee joints were collected by sectioning the femur and tibia/fibula 1 cm from the joint. Joints were fixed and decalcified using routine histologic procedures.
Experimental design: time course of wound healing following knee joint bleeding challenge
Three groups of mice were studied to establish the time course of histopathologic changes that occur in joint tissues over an 8-week period following articular exposure to blood in the joint puncture bleeding challenge. All mice received puncture injury of the left knee at day 0 to induce bleeding. Five microliters of normal saline (NS) was introduced into the joint space via the puncturing needle in groups of FIX−/− mice and WT C57Bl/6J mice (“WT NS” group). Citrated whole blood was collected from a third group of hemostatically normal C57Bl/6J mice, and each animal received 5 μL of its own fresh autologous blood injected into the joint at the same time that the wounding needle was introduced (“WT + Blood” group). Animals received no replacement coagulation factor. Separate groups of mice were euthanized for histopathologic examination on day 0 (unwounded) and on days 1, 3, 5, 7, 10, 14, 28, or 56 for the wound healing time course studies (4-8 mice per group per time point) or on day 14 in the treatment studies (7-8 mice per group).
rFIX proteins
Unmodified rFIX was BeneFIX (Pfizer, Philadelphia, PA). A dose-finding study was performed to determine the most informative dose of N9-GP (supplied by Novo Nordisk A/S, Maaloev, Denmark) for demonstrating differences (should they exist) in treatments to support wound healing. Details of this dose-finding study are included in the supplemental Methods and Results and supplemental Table 1 (available on the Blood Web site). In brief, a dose of N9-GP of ∼250 U/kg protected against severe synovitis in a majority of hemophilia B mice, but did not completely protect from mild synovial inflammation and other histopathologic abnormalities. Therefore, it was expected that this dose would allow quantifiable observation of a range of therapeutic responses to N9-GP. Additionally, the detailed comparative circulating pharmacokinetics of plasma FIX antigen and activity following dosing of N9-GP and rFIX in this strain of FIX−/− mice at this dose have been generated previously.22
Pharmacokinetic comparison of FIX measured in plasma and in synovial fluid
Comparative evaluation of FIX levels in plasma and synovial fluid was performed following administration of rFIX and N9-GP as per methods previously described8 and detailed in the supplemental Methods.
Experimental design: FIX treatment to support wound healing
Joint hemorrhage was induced in FIX−/− mice or WT mice. At 20 minutes after wounding, the FIX−/− mice were treated IV with either NS, with 250 IU/kg of N9-GP, or with 250 IU/kg of rFIX. WT mice received NS IV. Additional groups of mice received unmodified rFIX in repeated dose schedules after wounding. One group received 250 IU/kg of rFIX at the time of injury, on the following day, and again on the third day, equivalent to the “enhanced episodic” factor replacement schedule used in the Joint Outcome Study of Manco-Johnson et al.16 Another group of mice received a total of 8 replacement doses of rFIX given on an alternate-day schedule throughout 2 weeks following hemarthrosis. At 14 days after the hemarthrosis, mice were euthanized and tissues collected for histologic processing. Plasma FIX inhibitor testing was performed using the Bethesda inhibitor assay modified to detect human FIX in mouse plasma as previously described (lower level of sensitivity 1 Bethesda inhibitor unit per mL).24
Microscopic evaluation of synovium and cartilage
Sagittal sections of knee joints were prepared and stained with hematoxylin and eosin for synovitis grading. Hemophilic synovitis was graded in terms of synovial hyperplasia (0-3 points), vascularity (0-3 points), and the presence of discoloration, blood, villi, or cartilage erosion (0 or 1 point for each), resulting in a combined score of 0 to 10 points for increasing pathology, according to the system validated by Valentino and Hakobyan,25 as previously described.8,9 Additional sections were prepared with Prussian Blue stain for tissue iron and for immunohistochemical staining and grading for macrophages and vessels were counted by staining for endothelial cells by von Willebrand factor (VWF) as previously described by Hoffman et al6 with some modifications, as detailed in the supplemental Methods. For the time-course study only, joint cartilage pathology from Safranin-O–stained sections was graded to generate the Global Score of bleeding-induced joint pathology, as previously described by Narkbunnam et al9 (supplemental Figure 1). In light of the relatively small contribution of gross cartilage pathology to the Global Score, cartilage was additionally evaluated throughout the time course for total number of chondrocytes per high-powered field and for number of chondrocytes undergoing apoptosis per high-powered field.
Microcomputed tomography (microCT) evaluation of bone
Mice in the FIX treatment studies were euthanized on day 14 following hemarthrosis, and hind limbs were collected and fixed in formalin. Hind limbs were imaged using microCT at 10-µm resolution (µCT80; Scanco Medical AG, Brüttisellen, Switzerland). Analysis was performed on the trabecular bone of the injured limb inferior to the growth plate of the proximal tibia. Scanco analysis software was used to perform the histomorphometric analysis of trabecular morphology and bone mineral density (BMD), as previously described,26 and to derive a quantitative expression of the degree of disruption of the articular surface of the joint.
Statistics
The evolution of pathologic changes was examined by 2-way analysis of variance with Tukey’s adjustment for multiple comparisons (3 pairwise comparisons on each day). The effect of extended rFIX activity was examined by 1-way analysis of variance with Sidak’s adjustment for multiple comparisons (10 pairwise comparisons). An adjusted P value <.05 was considered a statistically significant difference. The analyses used version 6.00 for Windows (GraphPad Software, La Jolla, CA). The data are shown as the mean ± standard error of the mean.
Results
The evolution of pathologic changes following exposure of the joint to blood is different in hemostatically normal mice and hemophilia B mice
Joint bleeding was induced by needle puncture of the left knee joint capsule, as previously described,7-15 in cohorts of FIX−/− and hemostatically normal WT mice. To establish that mice of both hemostatic phenotypes did experience bleeding into the IA space following this challenge, groups of mice were euthanized at 2 hours after joint puncture, and the gross tissues were examined visually and the histology examined microscopically. Blood was observed in joints of both strains, although the amount of bleeding into the FIX−/− joints was greater than into the WT mouse joints (data not shown). Therefore, a third treatment group was added, composed of WT mice that experienced a puncture of the left knee joint capsule in an identical fashion to the other mice, but at the time of the needle puncture, 5 μL of freshly collected citrated autologous blood was injected into the joint to insure significant blood exposure (“WT + Blood” study group).
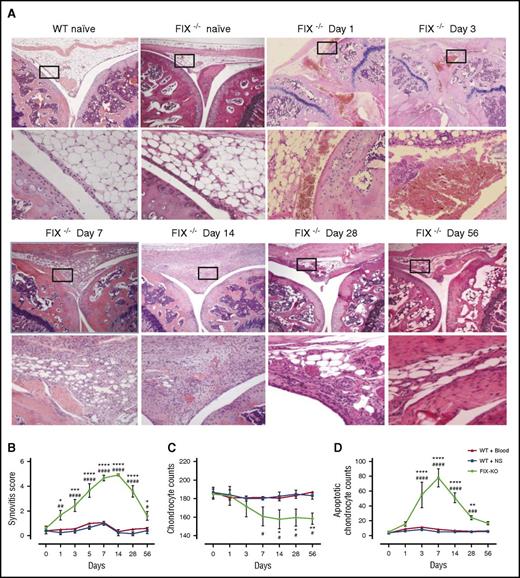
An 8-week time course of changes in the synovium and cartilage following hemarthrosis demonstrated different outcomes between hemophilic and hemostatically normal mice (supplemental Figure 1; Table 1). As early as 24 hours after injury, the clearance of blood from the synovial space in the WT and WT + Blood groups was nearly complete. This finding contrasts with the frank blood that dwelled in the joints of FIX−/− mice through at least day 3, by which time synovial pathology was evident (Figure 1A-B; supplemental Figure 2). Any minimal changes in synovial histology in both groups of mice with normal thrombin-generating potential were not evident after 1 week, whereas pathology did not resolve in hemophilia B mice throughout the 8 weeks of observation (Table 1; Figure 1A-B; supplemental Figure 2).
Evolution of IA pathology during 8-week time course of joint wound healing of FIX−/−mouse following hemarthrosis. (A) Representative histopathology. Images were captured on a Nikon Microphor SA microscope equipped with 10/0.30, 20/0.50, and 40/0.70 numeric aperture objective lenses. Photographs were taken with a DMX-1200 color camera using the Act-1 software (entire system from Nikon Instruments, Melville, NY). Representative images of joint histology for each condition/time point are shown at original magnification ×40 (top) and ×200 (bottom). WT naïve and FIX−/− naïve display normal histology of the uninjured mouse knee joint, with distal femur oriented on the left and proximal tibia on the right, with meniscus and synovial/subsynovial histology seen in the center, displaying normal 3-4 cell layer synovium overlying subsynovial fat with rare subsynovial blood vessels. Following hemarthrosis FIX−/− mouse histology is remarkable on day 1 only for frank blood within the joint space and in the subsynovial space. Day 3 thickening of the synovial lining is evident. By day 7, the subsynovial space is replaced by a dense collection of inflammatory infiltrate and proliferative synoviocytes, which is punctuated by day 14 with frequent neovascular structures. Synovial hyperplasia resolves in some (although not all) FIX−/− mice by day 56 following the single gross hemarthrosis; however, abnormal vascular changes persist. (B) Time course of synovial pathology. Joint capsule puncture was induced at day 0 in C57Bl/6J FIX−/− mice, in 2 separate cohorts of C57Bl/6J hemostatically normal (WT) mice. NS was injected into the joint at the time of needle puncture for one-half of the WT mice (WT + NS, ▪) and for all of the FIX−/− mice (FIX−/−, ●). The other half of the WT mice received autologous citrated whole blood injected into the joint at the time of needle puncture (WT + Blood, ▲). Separate groups of mice were euthanized for histopathologic examination of hematoxylin and eosin–stained joints at each time point during the 8-week time course. Hemophilic synovitis was graded 0 to 10 points for increasing pathology, according to the Valentino mouse hemophilic synovitis scale,25 which awards 0 to 10 points for increasing evidence of synovial overgrowth, neovascularity, and articular bleeding. Areas of greatest synovial thickening and vascularity were identified, and the average synovitis score from 3 nonoverlapping high-magnification fields was recorded. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, ***P < .001, ****P < .0001, #P < .05, ##P < .01, ####P < .0001. (C) Time course of total chondrocyte number. Total chondrocytes were counted in a blinded manner in 10 randomly selected hematoxylin and eosin–stained fields of femoral and tibial cartilage using a 20× objective lens. The mean and standard deviation for the entire cohort of animals at each time point is plotted. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, **P < .01, #P < .05. (D) Time course of apoptotic chondrocytes. Apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) using an In Situ Cell Death Detection Kit (Roche Diagnostics Corporation, Indianapolis, IN). TUNEL-positive cells were counted in a blinded manner in 10 randomly selected fields using a 20× objective lens. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: **P < .01, ****P < .0001, ###P < .001, ####P < .0001.
Evolution of IA pathology during 8-week time course of joint wound healing of FIX−/−mouse following hemarthrosis. (A) Representative histopathology. Images were captured on a Nikon Microphor SA microscope equipped with 10/0.30, 20/0.50, and 40/0.70 numeric aperture objective lenses. Photographs were taken with a DMX-1200 color camera using the Act-1 software (entire system from Nikon Instruments, Melville, NY). Representative images of joint histology for each condition/time point are shown at original magnification ×40 (top) and ×200 (bottom). WT naïve and FIX−/− naïve display normal histology of the uninjured mouse knee joint, with distal femur oriented on the left and proximal tibia on the right, with meniscus and synovial/subsynovial histology seen in the center, displaying normal 3-4 cell layer synovium overlying subsynovial fat with rare subsynovial blood vessels. Following hemarthrosis FIX−/− mouse histology is remarkable on day 1 only for frank blood within the joint space and in the subsynovial space. Day 3 thickening of the synovial lining is evident. By day 7, the subsynovial space is replaced by a dense collection of inflammatory infiltrate and proliferative synoviocytes, which is punctuated by day 14 with frequent neovascular structures. Synovial hyperplasia resolves in some (although not all) FIX−/− mice by day 56 following the single gross hemarthrosis; however, abnormal vascular changes persist. (B) Time course of synovial pathology. Joint capsule puncture was induced at day 0 in C57Bl/6J FIX−/− mice, in 2 separate cohorts of C57Bl/6J hemostatically normal (WT) mice. NS was injected into the joint at the time of needle puncture for one-half of the WT mice (WT + NS, ▪) and for all of the FIX−/− mice (FIX−/−, ●). The other half of the WT mice received autologous citrated whole blood injected into the joint at the time of needle puncture (WT + Blood, ▲). Separate groups of mice were euthanized for histopathologic examination of hematoxylin and eosin–stained joints at each time point during the 8-week time course. Hemophilic synovitis was graded 0 to 10 points for increasing pathology, according to the Valentino mouse hemophilic synovitis scale,25 which awards 0 to 10 points for increasing evidence of synovial overgrowth, neovascularity, and articular bleeding. Areas of greatest synovial thickening and vascularity were identified, and the average synovitis score from 3 nonoverlapping high-magnification fields was recorded. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, ***P < .001, ****P < .0001, #P < .05, ##P < .01, ####P < .0001. (C) Time course of total chondrocyte number. Total chondrocytes were counted in a blinded manner in 10 randomly selected hematoxylin and eosin–stained fields of femoral and tibial cartilage using a 20× objective lens. The mean and standard deviation for the entire cohort of animals at each time point is plotted. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, **P < .01, #P < .05. (D) Time course of apoptotic chondrocytes. Apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) using an In Situ Cell Death Detection Kit (Roche Diagnostics Corporation, Indianapolis, IN). TUNEL-positive cells were counted in a blinded manner in 10 randomly selected fields using a 20× objective lens. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: **P < .01, ****P < .0001, ###P < .001, ####P < .0001.
Acute cartilage insult was demonstrated by the finding of apoptosis within chondrocytes of the hemophilic mouse cartilage using TUNEL stain. Chondrocyte apoptosis peaked at 1 week after knee injury, by which time a loss of the total number of chondrocytes was observed. Resolution of apoptotic chondrocytes was not observed until after week 4 in the injured FIX−/− mice, so that chondrocyte counts did not recover by the end of 8 weeks of observation (Figure 1C-D). Neither group of injured hemostatically normal mice demonstrated chondrocyte changes.
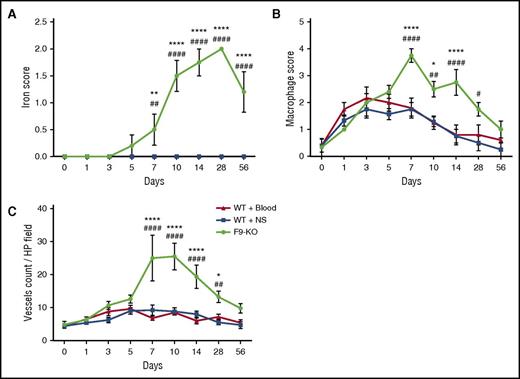
We performed a qualitative analysis of hemophilic joint wound healing following hemarthrosis using the same scales used by Hoffman et al6,27,28 (Table 1). Synovial iron deposition in FIX−/− mice was obvious beginning day 7 peaking at day 28 but never clearing throughout the 8 weeks of observation. There was no iron deposition in hemostatically normal mice at any time point, with iron from the injected autologous blood effectively cleared (Figure 2A). Macrophage/monocyte residence in the joint space peaked at days 3 to 7 in both groups of hemostatically normal mice and returned to baseline after the first week. The peak inflammatory cell response was delayed and ultimately more profound in hemophilic mice (Figure 2B). Neoangiogenesis was present in some individual mice of both the WT and hemophilic mouse groups (Figure 2C). Although the quantity of neovessels in the blood-exposed injured WT mouse joint was scant and not statistically significant, VWF-stained vessels were both more prominent and more persistent in the hemophilic mice.
Time course of joint wound healing parameters is abnormal within blood-exposed joint synovium in mice with hemophilia in comparison with hemostatically normal mice. (A) Iron staining in joint wounds from WT and FIX−/− mice. Iron released from degraded erythrocytes in the ferric (+3) state stains blue, heaviest areas of staining within joints of all mice were graded 0 to 3+, and the means and standard deviation for all cohorts of mice are plotted. Iron in hemoglobin is in the ferrous (+2) state so that intact red blood cells are not stained. Plots of the data for both groups of WT mice overlap completely. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: **P < .01, ****P < .0001, ##P < .01, ####P < .0001. (B) Macrophage infiltration and residence in joint wounds from WT and FIX−/− mice. Macrophage infiltrate was identified by immunostaining for CD68 and scored as described in the supplemental Methods. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, ****P < .0001, #P < .05, ##P < .01, ####P < .0001. (C) Angiogenesis in joint wounds in WT and FIX−/− mice. Vascular endothelial cells were identified by staining for VWF with a rabbit polyclonal primary antibody (Abcam, Cambridge, MA). The counts are expressed per high-powered (×40) field. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, ****P < .0001, ##P < .01, ####P < .0001.
Time course of joint wound healing parameters is abnormal within blood-exposed joint synovium in mice with hemophilia in comparison with hemostatically normal mice. (A) Iron staining in joint wounds from WT and FIX−/− mice. Iron released from degraded erythrocytes in the ferric (+3) state stains blue, heaviest areas of staining within joints of all mice were graded 0 to 3+, and the means and standard deviation for all cohorts of mice are plotted. Iron in hemoglobin is in the ferrous (+2) state so that intact red blood cells are not stained. Plots of the data for both groups of WT mice overlap completely. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: **P < .01, ****P < .0001, ##P < .01, ####P < .0001. (B) Macrophage infiltration and residence in joint wounds from WT and FIX−/− mice. Macrophage infiltrate was identified by immunostaining for CD68 and scored as described in the supplemental Methods. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, ****P < .0001, #P < .05, ##P < .01, ####P < .0001. (C) Angiogenesis in joint wounds in WT and FIX−/− mice. Vascular endothelial cells were identified by staining for VWF with a rabbit polyclonal primary antibody (Abcam, Cambridge, MA). The counts are expressed per high-powered (×40) field. Points that are significantly different from “WT + Blood” are indicated by “*.” Points that are significantly different from “WT + NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, ****P < .0001, ##P < .01, ####P < .0001.
Pharmacodynamics and pharmacokinetics of rFIX vs N9-GP in plasma and in joint
To establish the relative importance of initial and extended hemostatic support for healing following hemophilic joint bleeding, FIX replacement using unmodified rFIX was compared with FIX replacement using N9-GP. A dose-finding study of the effect of N9-GP given early in response to induced joint hemorrhage determined that comparing doses of 250 IU/kg of N9-GP and rFIX in subsequent studies would yield an informative range of therapeutic responses (supplemental Methods and Results and supplemental Table 1). Additionally, we examined whether monoPEGylation of FIX affected the accessibility of the protein to the intraarticular space. At 2 hours after IV administration of 250 IU/kg of either rFIX or N9-GP, the levels of FIX levels measured in the synovial fluid were similar (detailed in Table 2 and supplemental Methods and Results).
Extended FIX activity after hemarthrosis results in improved protection of synovium and cartilage
Joint hemorrhage was induced in FIX−/− or WT mice. At the time of euthanization, FIX inhibitors (≥1 Bethesda unit) were not present in plasma of any treated mice. None of the therapies given to hemophilic mice to interrupt ongoing hemorrhage (administered 20 minutes after wounding) resulted in joint health equivalent to that seen in the joints of the injured hemostatically normal mice (Figure 3A). Nevertheless, a single dose of N9-GP 250 IU/kg given following injury provided protection that was superior to a single dose of unmodified rFIX 250 IU/kg (P = .0111) and which was at least as good as the protection afforded by rFIX dosed according to the 2 repeated-dose regimens.
FIX therapy to support wound healing following hemarthrosis in the FIX−/−mouse joint. Joint hemorrhage was induced by needle puncture of the left knee joint capsule in all treatment groups, followed by tail vein infusion of first treatment or placebo (NS) at 20 minutes after wounding. Some treatment groups received repeated doses of rFIX at later time points as indicated. Treatment groups (6-8 mice/group) consisted of FIX−/− mice treated with a single dose of NS on day 0 (NS, ●); FIX−/− mice treated with a single dose of N9-GP on day 0 (N9-GP d0, ▪); FIX−/− mice treated with a single dose of rFIX on day 0 (rFIX d0, ▲); FIX−/− mice treated with rFIX on day 0, 1 and 3 (rFIX d0,1,3, ▼); FIX−/− mice treated with rFIX on day 0, 1,3,5,7,9,11,13 (rFIX d0-13, ♦); and WT mice treated with NS on day 0 (WT, ○). Points that are significantly different from “WT” are indicated by “*.” Points that are significantly different from “NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, **P < .01, ***P < .001, ****P < .0001, #P < .05, ##P < .01, ####P < .0001; fP < 0.05, ffP < 0.01. (A) Synovitis grade. As reported previously,8 untreated FIX−/− mice always demonstrate synovitis pathology graded greater than two-tenths on the Valentino scale at 2 weeks after this injury, whereas hemostatically normal mice never score greater than two-tenths. The dashed line indicates the historical threshold value of 2. FIX−/− mice that received N9-GP but not unmodified rFIX demonstrated mean synovitis score below this threshold of 2 out of 10. Apoptotic (TUNEL stain positive) chondrocytes (B) and total chondrocytes (C) were counted from femoral and tibial cartilage using a 20× objective lens, and the mean and standard deviation for the entire cohort of animals at each time point are plotted. (D) Iron released from degraded erythrocytes was observed as the intensity of Prussian blue staining ferric iron at ×40 magnification and was graded in the heaviest areas of synovial and subsynovial staining as described in the supplemental Methods. (E) Macrophage infiltration was identified by immunostaining for CD68 observed at ×40 magnification and graded in the most heavily infiltrated portion of synovium as described in the supplemental Methods. (F) Vascular endothelial cells were identified by staining for VWF observed at ×40 magnification and counted in the staining areas of the synovium and subsynovium.
FIX therapy to support wound healing following hemarthrosis in the FIX−/−mouse joint. Joint hemorrhage was induced by needle puncture of the left knee joint capsule in all treatment groups, followed by tail vein infusion of first treatment or placebo (NS) at 20 minutes after wounding. Some treatment groups received repeated doses of rFIX at later time points as indicated. Treatment groups (6-8 mice/group) consisted of FIX−/− mice treated with a single dose of NS on day 0 (NS, ●); FIX−/− mice treated with a single dose of N9-GP on day 0 (N9-GP d0, ▪); FIX−/− mice treated with a single dose of rFIX on day 0 (rFIX d0, ▲); FIX−/− mice treated with rFIX on day 0, 1 and 3 (rFIX d0,1,3, ▼); FIX−/− mice treated with rFIX on day 0, 1,3,5,7,9,11,13 (rFIX d0-13, ♦); and WT mice treated with NS on day 0 (WT, ○). Points that are significantly different from “WT” are indicated by “*.” Points that are significantly different from “NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, **P < .01, ***P < .001, ****P < .0001, #P < .05, ##P < .01, ####P < .0001; fP < 0.05, ffP < 0.01. (A) Synovitis grade. As reported previously,8 untreated FIX−/− mice always demonstrate synovitis pathology graded greater than two-tenths on the Valentino scale at 2 weeks after this injury, whereas hemostatically normal mice never score greater than two-tenths. The dashed line indicates the historical threshold value of 2. FIX−/− mice that received N9-GP but not unmodified rFIX demonstrated mean synovitis score below this threshold of 2 out of 10. Apoptotic (TUNEL stain positive) chondrocytes (B) and total chondrocytes (C) were counted from femoral and tibial cartilage using a 20× objective lens, and the mean and standard deviation for the entire cohort of animals at each time point are plotted. (D) Iron released from degraded erythrocytes was observed as the intensity of Prussian blue staining ferric iron at ×40 magnification and was graded in the heaviest areas of synovial and subsynovial staining as described in the supplemental Methods. (E) Macrophage infiltration was identified by immunostaining for CD68 observed at ×40 magnification and graded in the most heavily infiltrated portion of synovium as described in the supplemental Methods. (F) Vascular endothelial cells were identified by staining for VWF observed at ×40 magnification and counted in the staining areas of the synovium and subsynovium.
Consistent with the time course described previously, the cartilage of NS placebo-treated FIX−/− mice demonstrated more apoptotic chondrocytes and a lower mean chondrocyte number than cartilage of NS-treated hemostatically normal mice at 2 weeks after injury (Figure 3B-C). All FIX replacement regimens provided some chondrocyte protection. A trend toward increased chondrocyte cell death in the mice that received a single dose of rFIX was not, however, significantly different from WT mice and not associated with significantly diminished chondrocyte counts at 2 weeks following injury. A single dose of N9-GP or a 3- or 8-dose regimen of rFIX resulted in protection from chondrocyte apoptosis to a level similar to injured WT mice.
Effect of extended FIX activity on iron deposition, inflammation, and neovascularity following hemarthrosis
The heavy iron staining observed at 2 weeks after hemarthrosis in hemophilic mice (Figure 3D; supplemental Figure 4B) was improved by rFIX therapy (Figure 3D). A single dose of rFIX did not prevent the iron deposition being statistically greater than that observed in hemostatically normal mice. Conversely, intensified repetitive-dose rFIX regimen or a single dose of N9-GP prevented iron deposition in the joints to a level that was not statistically distinguishable from that observed in hemostatically normal mice (Figure 3D; supplemental Figure 4B). Similar patterns of wound healing were observed in regard to synovial macrophage residence and neoangiogenesis, with single-dose N9-GP resulting in healing similar to multidose rFIX therapy (Figure 3E-F).
Extended FIX activity after hemarthrosis and preservation of bone density, structure, and the integrity of the articulating joint surface
We have previously reported that hemophilia A mice, examined by microCT scan, exhibit ectopic mineralization and roughening of the surface of the articulating bones following hemarthrosis.26 Hemophilia B mice in this study exhibited these same acute changes involving distal femur, proximal tibia, and patella (Figure 4A). Following the induced hemarthrosis, joints from animals treated with N9-GP demonstrated preservation of articular surface smoothness that was not statistically significantly different from that seen in hemostatically normal WT mice. rFIX was less effective at preserving the integrity of the surface of the articulating bones (Figure 4B).
Abnormal bone wound healing and FIX replacement to maintain health of bones following hemarthrosis in normal and in FIX−/−mice. (A) Preservation of articulating surface of joint following hemarthrosis (microCT images). Three-dimensional rendering of the injured knee joint from microCT imaging obtained at 2 weeks after a single induced hemarthrosis followed by a single dose of placebo or FIX therapy at 20 minutes after wounding. WT: Hemostatically normal mouse, sham treated with IV NS. FIX−/−: Hemophilia B mouse sham treated with IV NS. FIX−/− + rFIX: Hemophilia B mouse treated with rFIX (Benefix) 250 IU/kg IV. FIX−/− + N9-GP: Hemophilia B mouse treated with glycoPEGylated rFIX (N9-GP) 250 IU/kg IV. (B) Quantification of loss of integrity of articulating surface of the joint following hemarthrosis (“ratio of smoothness”). To quantify the observed loss of surface integrity, a mathematical model was derived from the imaged joints that calculated the shape of a theoretically perfectly smooth joint surface, which was assigned a value of 1.0. Any imaged depressions or elevations resulted in deviations from absolute smoothness, resulting in values of <1.0, giving a measurement of “ratio of smoothness” that is less than the ideal. At 2 weeks after the induced hemarthrosis, joints from animals treated with N9-GP demonstrated preservation of articular surface smoothness that was not statistically significantly different from that seen in hemostatically normal WT mice (ratio = 0.901 and 0.906, respectively). rFIX was less effective at preserving the integrity of the surface of the articulating bones. Points that are significantly different from “WT” are indicated by “*.” Points that are significantly different from “NS” are indicated by “#.” Markers of significance are used as follows: **P < .01, ****P < .0001, ###P < .001, ####P < .0001.
Abnormal bone wound healing and FIX replacement to maintain health of bones following hemarthrosis in normal and in FIX−/−mice. (A) Preservation of articulating surface of joint following hemarthrosis (microCT images). Three-dimensional rendering of the injured knee joint from microCT imaging obtained at 2 weeks after a single induced hemarthrosis followed by a single dose of placebo or FIX therapy at 20 minutes after wounding. WT: Hemostatically normal mouse, sham treated with IV NS. FIX−/−: Hemophilia B mouse sham treated with IV NS. FIX−/− + rFIX: Hemophilia B mouse treated with rFIX (Benefix) 250 IU/kg IV. FIX−/− + N9-GP: Hemophilia B mouse treated with glycoPEGylated rFIX (N9-GP) 250 IU/kg IV. (B) Quantification of loss of integrity of articulating surface of the joint following hemarthrosis (“ratio of smoothness”). To quantify the observed loss of surface integrity, a mathematical model was derived from the imaged joints that calculated the shape of a theoretically perfectly smooth joint surface, which was assigned a value of 1.0. Any imaged depressions or elevations resulted in deviations from absolute smoothness, resulting in values of <1.0, giving a measurement of “ratio of smoothness” that is less than the ideal. At 2 weeks after the induced hemarthrosis, joints from animals treated with N9-GP demonstrated preservation of articular surface smoothness that was not statistically significantly different from that seen in hemostatically normal WT mice (ratio = 0.901 and 0.906, respectively). rFIX was less effective at preserving the integrity of the surface of the articulating bones. Points that are significantly different from “WT” are indicated by “*.” Points that are significantly different from “NS” are indicated by “#.” Markers of significance are used as follows: **P < .01, ****P < .0001, ###P < .001, ####P < .0001.
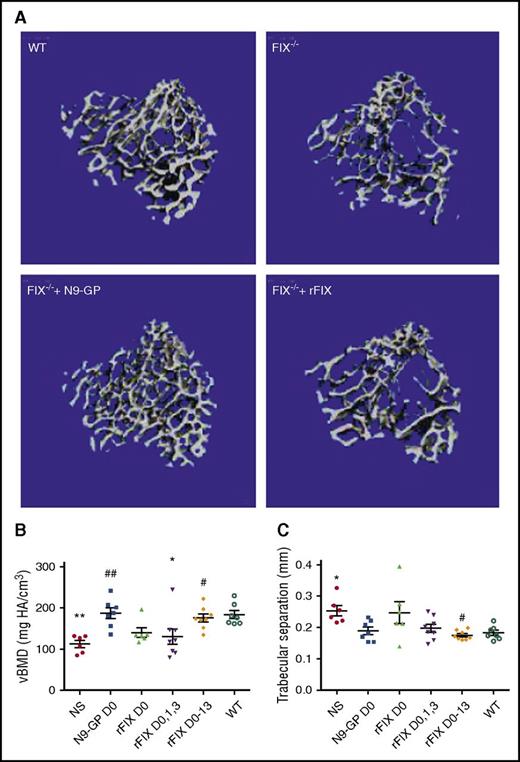
The untreated hemophilia B mice in this study also experienced an acute loss of greater than one-third of the volumetric bone mineral density (vBMD) measured at the proximal tibia adjacent to the blood-exposed knee when compared with hemostatically normal mice (Figure 5A-B). The vBMD measures the average mineral density over the entire trabecular compartment, including the space between the bone tissue, and so this microCT measurement is similar to that of a clinical dual-energy X-ray densitometry bone density scan but performed in 3-dimensional space. Mice given rFIX for 1 to 4 days after wounding exhibited diminished BMD, which was, however, normalized by 8-dose rFIX treatment during the 2 weeks of healing. Loss of BMD was not observed in mice treated with single-dose N9-GP following hemarthrosis.
Abnormal trabecular bone health and FIX to support bone preservation following hemarthrosis in normal and in FIX−/−mice. (A) Experimental animals include the same conditions as in Figure 4, that is, 2-week postinjury images from sham-treated WT, sham-treated FIX−/−, and hemophilia B mice treated at 20 minutes after wounding with a single dose of 250 IU/kg IV rFIX (Benefix) FIX−/− + rFIX or a single dose of 250 IU/kg IV glycoPEGylated rFIX (N9-GP) FIX−/− + N9-GP. Images of the trabecular bone region of analysis of the proximal tibia demonstrate less bone in the representative image from the injured, placebo-treated hemophilia B mouse when compared with the WT placebo-treated mouse, as well as relatively less good preservation of bone density by single-dose rFIX in comparison with N9-GP. (B) Quantitative analysis of vBMD outcomes at 2 weeks following injury. Although large variations between mice were seen within each group, single-dose rFIX only partially preserved connectivity density of the trabecular struts, whereas the N9-GP or the 2 week replacement with rFIX preserved connectivity. (C) Trabecular bone microarchitecture changes following hemarthrosis include increase in trabecular separation in hemophilia mice compared with mice with intact hemostasis. Mean trabecular separation was preserved following hemarthrosis (not different from WT) by each of the replacement rFIX and N9-GP approaches. Normal trabecular separation was preserved by a single dose of N9-GP but required multiple dose rFIX therapy. Points that are significantly different from “WT” are indicated by “*.” Points that are significantly different from “NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, **P < .01, #P < .05, ##P < .01.
Abnormal trabecular bone health and FIX to support bone preservation following hemarthrosis in normal and in FIX−/−mice. (A) Experimental animals include the same conditions as in Figure 4, that is, 2-week postinjury images from sham-treated WT, sham-treated FIX−/−, and hemophilia B mice treated at 20 minutes after wounding with a single dose of 250 IU/kg IV rFIX (Benefix) FIX−/− + rFIX or a single dose of 250 IU/kg IV glycoPEGylated rFIX (N9-GP) FIX−/− + N9-GP. Images of the trabecular bone region of analysis of the proximal tibia demonstrate less bone in the representative image from the injured, placebo-treated hemophilia B mouse when compared with the WT placebo-treated mouse, as well as relatively less good preservation of bone density by single-dose rFIX in comparison with N9-GP. (B) Quantitative analysis of vBMD outcomes at 2 weeks following injury. Although large variations between mice were seen within each group, single-dose rFIX only partially preserved connectivity density of the trabecular struts, whereas the N9-GP or the 2 week replacement with rFIX preserved connectivity. (C) Trabecular bone microarchitecture changes following hemarthrosis include increase in trabecular separation in hemophilia mice compared with mice with intact hemostasis. Mean trabecular separation was preserved following hemarthrosis (not different from WT) by each of the replacement rFIX and N9-GP approaches. Normal trabecular separation was preserved by a single dose of N9-GP but required multiple dose rFIX therapy. Points that are significantly different from “WT” are indicated by “*.” Points that are significantly different from “NS” are indicated by “#.” Markers of significance are used as follows: *P < .05, **P < .01, #P < .05, ##P < .01.
The trabecular connectivity density, separation, and number are all structural microarchitectural parameters that may correlate with bone strength. At 2 weeks postinjury, in comparison with WT animals, untreated FIX−/− group displayed a 28% increase in trabecular spacing (supplemental Table 2), consistent with diminished bone strength. Smaller trends regarding loss of trabecular number and connectivity density in FIX−/− mice did not reach statistical significance. Normal trabecular bone properties were best preserved by rFIX D0-13 or N9-GP D0 replacement therapy.
Discussion
The induced joint hemorrhage model in hemophilia mice has been widely used to examine histopathologic and molecular changes following hemophilic joint hemorrhage, as well as therapeutic interventions. We examined the hypothesis that joint wound healing following a single bleeding exposure is qualitatively abnormal in the absence of normal thrombin generation. We show that wound healing following bleeding into joints of hemophilia B mice is impaired. Most strikingly, the absence of normal hemostatic potential is associated with a diminished capacity to clear blood and heme iron from the joint space. The abnormal morphologic parameters seen here following hemophilic joint bleeding closely parallel those previously demonstrated in another hemophilic healing model of a dermal wound.6,27,28
Following the induced joint wound, the animals with normal hemostasis (with or without the additional applied blood load) demonstrate only modest inflammatory cellular, synovial proliferative, and angiogenic changes followed by orderly resolution. (Table 1; Figures 1B and 2; supplemental Figure 2). In the absence of coagulation FIX, the angiogenic and proliferative changes that occur are pathologic rather than physiologic in degree, and the resolution of these processes is delayed or incomplete at the end 8 weeks’ observation. The time course of neoangiogenesis and vascular remodeling within the FIX−/− mouse joint that we describe are very consistent with changes described in a careful evaluation of the same hemarthrosis model performed in FVIII−/− mice by Bhat et al.29 Other processes that have no physiologic role in wound healing (eg, heme iron deposition, chondrocyte apoptosis) do not occur following wounding in the nonhemophilic animals (even when challenged with an applied blood load) but are prominent in hemophilia.
The current study demonstrates for the first time that induced joint bleeding in hemophilia B mice is associated with disrupted bone homeostasis, including diminished vBMD of trabecular bone and heterotopic mineralization of the external surface of the bones adjacent to hemarthrosis not seen in hemostatically normal mice that experience the same injury. These findings are consistent with our previous observations in hemophilia A mice.26 The deficits observed following hemarthrosis may compound a congenital tendency to low BMD that has been described in hemophilic mice.30,31 These animal findings are of particular interest in light of increasing awareness of the high prevalence of low BMD in individuals with hemophilia A and B.32-38 In addition, high-resolution peripheral quantitative CT examination of an adult hemophilia population has recently correlated more severe hemophilic arthropathy with low BMD.39
The consistency of the findings of abnormal wound healing between the hemophilic incisional wound model and the joint wound model suggests that studies of the pathophysiology and therapy of hemophilic joint disease that are performed in hemostatically normal animals should be interpreted with caution. Attempts to reproduce hemophilic arthropathy by modeling repeated joint bleedings in hemostatically normal dogs,2 or with repeated autologous intra-articular blood injections in nonhemophilic rats,3 led investigators to conclude that blood exposure alone could not lead to hemophilic joint damage.2,3 This observation is strikingly different from the gross pathology that develops following spontaneous joint bleeding in hemophilic dogs,40,41 hemophilic rats,42 and following 3 induced joint hemorrhages in hemophilic mice.9 This is further corroborated by our observation that blood injected into the joint of WT animals is cleared efficiently.
Thrombin serves many functions beyond hemostasis, so that absent thrombin generation may contribute to abnormal joint wound healing via additional mechanisms beyond joint exposure to hemorrhage. During normal cutaneous wound healing, thrombin promotes the physiologic neoangiogenesis and fibroblast migration that are required for orderly remodeling. Thrombin and thrombin signaling through protease activated receptor 1 have been reported to enhance incisional wound healing.43 The absence of thrombin may contribute to the disorganized and pathologic neoangiogenesis and fibroblast infiltration in the blood-exposed hemophilic joint. In the absence of thrombin, protein C activation is impaired; anti-inflammatory actions of activated protein C to protect the wounded joint would be lost and neutrophil infiltration unchecked.44,45
Absent thrombin generation in hemophilia, as well as the coincident loss of activated protein C activation by thrombin, translates to loss of osteoblast stimulation and unopposed inhibition of osteoblast apoptosis and may contribute to impaired bone remodeling after wounding.46-49 Additionally, plasmin and fibrinolysis may be directly toxic to cartilage. Normal activation of thrombin-activatable fibrinolysis inhibitor results in diminished formation of plasmin, attenuation of fibrinolysis, increased clot stability, and anti-inflammatory effects that may protect the healing joint wound.13,50,51 However, relatively high concentrations of thrombin are required to activate thrombin-activatable fibrinolysis inhibitor in the low tissue factor environment within the joint.51 In summary, absent thrombin generation in hemophilia potentially disrupts normal joint remodeling in synovium, cartilage, and bone by multiple mechanisms that compound the direct mechanical and toxic effects of hemorrhage.
A limitation of these studies is that the FIX−/− mice rarely demonstrate spontaneous bleeding. There is, however, no evidence that the body has different mechanisms for healing traumatic hemarthroses when compared with spontaneous hemarthroses. Another possible limitation is the attempt to model the healing response to an autologous blood load in hemostatically normal mice. Exposure of the joint to citrated blood may not recapitulate the blood challenge that occurs during traumatic clinical intraarticular hemorrhage in individuals who do not have hemophilia. The volume of blood injected (5 μL) was chosen based on our work suggesting this amount of blood will fill the mouse joint space (data not shown) and demonstrates a clear difference in the clearance of blood from the joint in the presence (compared with the absence) of normal hemostasis. It is possible that the WT mouse joint challenged with a broad range of injected blood loads might demonstrate different healing outcomes, although it would be difficult to interpret the relative contributions of barotrauma, hypoxia, and differing load of blood components to the observed pathology.
Restoring hemostatic potential only until hemostasis was obtained did not result in optimal joint wound healing. Instead, optimal joint health is achieved in this model by extending clotting factor activity during wound healing.27,28,52
The use of N9-GP, which is an FIX engineered for prolonged survival in circulation, resulted in improved wound healing when compared with single or repeated doses of rFIX. Extending hemostatic activity during wound healing by single-dose administration of N9-GP resulted in healing that was statistically not different from the healing seen in WT mice in regard to iron deposition and BMD. Mice that received rFIX administered in the “enhanced episodic” schedule of 3 doses of rFIX nevertheless healed less well than WT mice with regard to bone health, experiencing diminished vBMD and increased surface roughening. rFIX infused throughout 2 weeks of healing resulted in correction of vBMD (articular surface irregularity was not prevented). Notably, mice treated with N9-GP on day 0 and day 7 demonstrated no significant improvement in any parameters when compared with mice that received N9-GP on day 0 alone (supplemental Figure 4). The pharmacokinetics and pharmacodynamics of a single dose of N9-GP in hemophilia B mice given at the dose used in this study are well established. This dose provides circulating activity (>5% to 10% of normal) and clotting during bleeding and thrombotic challenges for at least 5 to 7 days,22 which appears to be the critical period for optimal joint and bone outcomes in this gross hemarthrosis model. We are currently investigating the possibility that prophylactic rFIX or FIX engineered to have extended circulating kinetics (N9-GP) can achieve fully normal joint healing following hemarthrosis that is equivalent to healing in hemostatically normal mice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge the outstanding technical assistance, consultation, and discussion of Elizabeth P. Chappell, Jacqueline B. Mickelson, Anthony G. Lau, and Tom Knudsen.
This work was supported in part by research funding from Novo Nordisk (D.M.M. and P.E.M.).
Authorship
Contribution: J.S. performed the studies, generated and adapted “Methods,” prepared the figures, and reviewed and revised the manuscript; B.H. performed the studies and reviewed and revised the manuscript; E.W.L. performed the studies, adapted “Methods,” prepared the figures, and reviewed and revised the manuscript; S.T. reviewed and revised the manuscript, prepared the figures, and performed statistical analysis; P.B.J. performed statistical analysis; M.H. and M.E. provided a critical review of the study design and edited the manuscript; D.M.M. provided a critical review of the study design, assisted the studies, and edited the manuscript; T.A.B. designed and oversaw the studies, reviewed and interpreted the data, and edited the manuscript; and P.E.M. designed and oversaw all studies, interpreted all data, and wrote the manuscript.
Conflict-of-interest disclosure: P.E.M. during the conduct of these studies received research support through the University of North Carolina from Asklepios BioPharmaceutical and Novo Nordisk. He has received research support in the past from Baxter Healthcare, Novo Nordisk, Pfizer, and Prolor. He holds patents licensed to Asklepios, for which he receives royalties. He has received payment for consultation, services, and for speaking for Asklepios, Chatham LLC, Baxter Healthcare, and Pfizer and has additionally consulted for Bayer, Novo Nordisk, and Biogen. Following the completion of these studies, but during a portion of the time of manuscript preparation, he has been an employee of Baxalta (now Shire). B.H. has received research support from the Novo Nordisk Haemophilia Research Fund following his active participation in the reported research. M.H. reports receiving grants from Novo Nordisk during the conduct of the study and grants from CSL Behring and Boehringer Ingelheim outside the submitted work. M.E. is an employee of Novo Nordisk. D.M.M. reports receiving grants, personal fees, and nonfinancial support from Novo Nordisk during the conduct of the study and grants from Novo Nordisk outside the submitted work. T.A.B. has received remuneration for consulting and speaker work for Baxter Health Care Corporation and research support from Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: Paul E. Monahan, University of North Carolina, Chapel Hill, 141 Graylyn Dr, Chapel Hill, NC 27516; e-mail: pablomonoloco@gmail.com.
References
Author notes
J.S. and B.H. contributed equally to this study.