Key Points
Mice expressing a form of prothrombin with limited activation potential to meizothrombin are viable and are reproductively successful.
Meizothrombin directly activates platelets but has diminished positive regulation of hemostatic factor activation.
Abstract
Thrombin-mediated proteolysis is central to hemostatic function but also plays a prominent role in multiple disease processes. The proteolytic conversion of fII to α-thrombin (fIIa) by the prothrombinase complex occurs through 2 parallel pathways: (1) the inactive intermediate, prethrombin; or (2) the proteolytically active intermediate, meizothrombin (fIIaMZ). FIIaMZ has distinct catalytic properties relative to fIIa, including diminished fibrinogen cleavage and increased protein C activation. Thus, fII activation may differentially influence hemostasis and disease depending on the pathway of activation. To determine the in vivo physiologic and pathologic consequences of restricting thrombin generation to fIIaMZ, mutations were introduced into the endogenous fII gene, resulting in expression of prothrombin carrying 3 amino acid substitutions (R157A, R268A, and K281A) to limit activation events to yield only fIIaMZ. Homozygous fIIMZ mice are viable, express fII levels comparable with fIIWT mice, and have reproductive success. Although in vitro studies revealed delayed generation of fIIaMZ enzyme activity, platelet aggregation by fIIMZ is similar to fIIWT. Consistent with prior analyses of human fIIaMZ, significant prolongation of clotting times was observed for fIIMZ plasma. Adult fIIMZ animals displayed significantly compromised hemostasis in tail bleeding assays, but did not demonstrate overt bleeding. More notably, fIIMZ mice had 2 significant phenotypic advantages over fIIWT animals: protection from occlusive thrombosis after arterial injury and markedly diminished metastatic potential in a setting of experimental tumor metastasis to the lung. Thus, these novel animals will provide a valuable tool to assess the role of both fIIa and fIIaMZ in vivo.
Introduction
The activation of prothrombin (fII) is the penultimate step of hemostasis. Thrombin (fIIa) cleaves fibrinogen and directly activates platelets, via protease-activated receptors (PARs).1 However, fIIa also controls its own production, via activation of factors V (fV), VIII (fVIII), XI (fXI), and protein C.2 Through these targets and others (eg, factor XIII [fXIII], thrombin-activatable fibrinolysis inhibitor), fIIa not only plays a pivotal role in hemostasis, but in other physiologic and pathologic processes (eg, development, inflammation, cancer biology).3-7 Mouse studies have underlined the seminal importance of fII, because the genetic elimination of fII is not compatible with life.3,4,8
Prothrombin activation proceeds through 2 parallel activation pathways, depending on the site of first cleavage by the prothrombinase complex. One pathway features an intermediate that is not an active enzyme, prethrombin, whereas the second pathway is via an active enzyme precursor, meizothrombin (fIIaMZ).9-11 FIIaMZ, as an active enzyme, is capable of participating in hemostasis and thrombosis, as well as other physiologic processes. FIIaMZ, like fIIa, activates fV,12 fVIII,13 and fXI.14 Thus, fIIMZ may contribute to positive feedback of physiologic hemostasis. Both human and murine rfIIaMZ demonstrate reduced fibrinogen cleavage capacity.15,16 Further, rfIIaMZ from both species demonstrates an increased activation potential for protein C, in the presence of thrombomodulin. Thus, fIIaMZ has potentially distinct effects on hemostasis regulation from that of the mature fIIa enzyme. At least 2 described human mutations result in a partial limitation of the activation of prothrombin to meizothrombin. Both prothrombin-Dharhan (R271H)17 and prothrombin-Barcelona/Madrid (R271C)18-21 limit fII activation to meizothrombin-like enzymes by removing a cleavage site. For each of the described pedigrees, individuals that were heterozygotes did not have a bleeding diathesis, whereas homozygotes had a mild to moderate bleeding tendency. Thus, although limitation of fII activation has a clear hemostatic effect, the broader biological impact of meizothrombin in vivo has not been defined. The fact that the spontaneous human mutations were viable in a homozygous state supports the feasibility for examining meizothrombin in a homozygous state in mice.
To better understand the biology of fII overall, and fIIaMZ, specifically, we generated a mouse with knock-in targeted mutations in the endogenous F2 allele, resulting in 3 amino acid substitutions (R157A, R258A, and K281A) that lock fII after activation by factor Xa (fXa) to meizothrombin. These mutations in murine fII have previously been identified to limit activation to meizothrombin.16 The working hypothesis was that mice expressing a form of fII that is limited to activation to fIIaMZ, would (1) be viable without spontaneous hemorrhage, (2) allow examination of the role of fIIaMZ in hemostasis in vivo, and (3) provide a novel tool for study of the biology of prothrombin in physiologic and pathologic conditions.
Methods
Generation of fIIMZ gene–targeted mice
For details of the generation of fIIMZ gene–targeted mice, please see the supplemental Methods, available on the Blood Web site. Mice that are heterozygous for the fII null allele (fII+/−) and compound heterozygotes for the conditional fII allele and fII null (fIIlox/–) were previously described.8 In vivo and ex vivo (ie, prothrombin time [PT], activated partial thromboplastin time [aPTT], and quantitative polymerase chain reaction [qPCR]) experiments used outbred hybrid (129/Ola:C57/Bl6) littermate controls, except as noted using at least 6 generations–inbred C57/Bl6 animals. All experiments were approved by the Cincinnati Children’s Hospital Research Foundation Animal Care and Use Committee and complied with National Institutes of Health guidelines.
Hematologic profile, determination of prothrombin levels, thrombin generation assay, and bleeding times
For details of the hematologic analysis, please see the supplemental Methods.
Prothrombin activation assay
For the prothrombin activation assay, citrated platelet-poor plasma was diluted 1:10 with sterile Tris-buffered saline. The peptide Gly-Pro-Arg-Pro (EMD Millipore) was added to a final concentration of 4 mM to prevent fibrin polymerization. Thromboplastin (Diapharma), reconstituted in sterile water, was added to the plasma. Aliquots were taken from this solution and directly added to sodium dodecyl sulfate sample buffer to stop the reaction, at time points as indicated in Results. Immunoblots of these samples were performed under reducing conditions, using an antibody specific for the N-terminus of prothrombin (antigen was a peptide within the first 50 amino acids of prothrombin, sc-23340, Santa Cruz Biotechnology). Detection would yield, in decreasing size, intact prothrombin, F1.2.A, F1.2, or F1.
Thromboplastin-induced platelet aggregation and clot retraction
Please see the supplemental Methods.
Real-time analysis of in vivo thrombus formation by intravital microscopy
Cohorts of fIIWT and fIIMZ mice were infused with genotype-controlled, fluorescently-labeled platelets. Anesthetized animals then had mesenteric arterioles isolated, and 10% topical ferric chloride solution was applied for 5 minutes. An observer blinded to genotype monitored vessels for the time to the first thrombus formation and the time until occlusion or for 30 minutes.
Lipopolysaccharide challenge and activated protein C levels
Please see the supplemental Methods.
Histopathology
Tissues were fixed in 10% buffered formalin (Sigma) and embedded in paraffin. Sections were cut and subsequently stained with hematoxylin and eosin (Sigma), Masson’s trichrome, or Prussian blue (Electron Microscopy Services). Photomicrographs were captured using an Axioplan 2 microscope (Zeiss) equipped with an AxiocamHR camera and software (Axiovision 5.6.3; Zeiss).
Experimental tumor metastasis
As described,22 a single-cell suspension of 4 × 105 GFP-expressing Lewis lung carcinoma cells (LLCGFP) was injected IV in cohorts of C57/Bl6 fIIWT and fIIMZ mice in parallel. Fluorescent pulmonary metastatic foci were evaluated 14 days after injection. For B16 melanoma experimental metastases, a single-cell suspension was injected (8 × 104 cells) IV into cohorts of C57/Bl6 fIIWT, fIIMZ, and fII+/− mice in parallel, as previously described.23 Nineteen days after injection, lungs were removed, stained overnight in picric acid solution (Sigma), and the metastatic foci counted.
Statistical analysis
All statistical analyses were generated using the Mann-Whitney U test, except as follows: the χ2 test was used to analyze breeding data and Fisher’s exact test was used to analyze the tail bleeding times and percent occlusion in intravital microscopy experiments. Statistical analyses were performed on GraphPad Prism version 5.04 (GraphPad Software).
Results
Mice carrying the fIIMZ allele
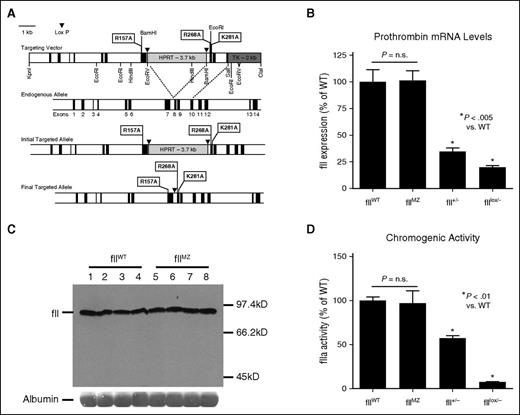
To determine the in vivo consequences of expressing a form of prothrombin incapable of activation beyond the intermediate, meizothrombin, we introduced 3 amino acid substitutions into the endogenous allele, to remove both a fXa cleavage site and potential fIIa auto-cleavage sites: R157A, R268A, and K281A (Figure 1A).24 The R157A and R268A cleavage site mutations have been previously identified to limit murine prothrombin activation to meizothrombin.16 In addition to these 3 amino acid changes, we introduced a novel BamHI endonuclease site in Exon 7 and a novel EcoRI site in Exon 8, without further modification of the amino acid coding sequence. Founder mice transmitted the mutant allele through the germline to yield mice heterozygous for the mutation (fIIMZ/WT). Analyses of intercrosses of fIIMZ/WT mice resulting in >800 progeny revealed an ∼50% decrease in the numbers of homozygous mutant animals (hereafter referred to as fIIMZ) relative to what would be expected based on a 1:2:1 Mendelian ratio (Table 1). Further studies were conducted to confirm the timing of failure of the homozygous animals. Analyses of embryos harvested at embryonic day 18.5 (E18.5; ie, the day before birth) from fIIMZ/WT breeding pairs did not reveal a significant difference in relative numbers of fIIWT and fIIMZ embryos (Table 2). This suggests the loss of homozygous fIIMZ animals occurs in the early postnatal timeframe. Consistent with this conclusion, evidence of abdominal hemorrhage in fIIMZ pups was observed (supplemental Figure 1). Homozygous fIIMZ mice identified at weaning survived well into adulthood without excess mortality. Of the >200 adult fIIMZ mice generated to date, none were found to suffer overt hemorrhage. Furthermore, homozygous fIIMZ females were capable of carrying litters to term, with 23 C57Bl/6 homozygous fIIMZ females successfully carrying 52 litters to term.
Alteration of the endogenous fII allele to limit activation to meizothrombin. (A) This map demonstrates the targeting vector used to alter the endogenous fII allele as well as a map of the endogenous allele. Incorporation of the targeting vector by homologous recombination targeting vector was confirmed in germline-competent mice by PCR. After transmission of the targeted allele by the chimeric animals, mice were crossed with CMV-Cre animals to remove the HPRT cassette, with the final targeted allele as illustrated. Loss of the HPRT cassette was also confirmed by PCR. (B) Liver fII mRNA levels in fIIWT and fIIMZ animals were assessed using qPCR. No significant difference in fII RNA levels was detected between the 2 genotypes. In contrast, significant decreases, as expected, were noted in fII+/− and fIIlox/− animals (n = 6 in all cohorts). (C) Plasma from both fIIMZ and fIIWT mice was assayed for fII antigen levels via western blot. No difference in fII levels between fIIWT and fIIMZ animals was appreciable on the immunoblot. (D) Chromogenic fIIa activity from both fIIWT and fIIMZ plasma was assayed after activation with ecarin. There was no significant difference in plasma fIIa activity derived from either fIIMZ or fIIWT animals (n = 7 in both cohorts). The expected decrease in fIIa activity in both fII+/− and fIIlox/− (n = 4 in both cohorts) was readily detectable.
Alteration of the endogenous fII allele to limit activation to meizothrombin. (A) This map demonstrates the targeting vector used to alter the endogenous fII allele as well as a map of the endogenous allele. Incorporation of the targeting vector by homologous recombination targeting vector was confirmed in germline-competent mice by PCR. After transmission of the targeted allele by the chimeric animals, mice were crossed with CMV-Cre animals to remove the HPRT cassette, with the final targeted allele as illustrated. Loss of the HPRT cassette was also confirmed by PCR. (B) Liver fII mRNA levels in fIIWT and fIIMZ animals were assessed using qPCR. No significant difference in fII RNA levels was detected between the 2 genotypes. In contrast, significant decreases, as expected, were noted in fII+/− and fIIlox/− animals (n = 6 in all cohorts). (C) Plasma from both fIIMZ and fIIWT mice was assayed for fII antigen levels via western blot. No difference in fII levels between fIIWT and fIIMZ animals was appreciable on the immunoblot. (D) Chromogenic fIIa activity from both fIIWT and fIIMZ plasma was assayed after activation with ecarin. There was no significant difference in plasma fIIa activity derived from either fIIMZ or fIIWT animals (n = 7 in both cohorts). The expected decrease in fIIa activity in both fII+/− and fIIlox/− (n = 4 in both cohorts) was readily detectable.
Prothrombin expression levels were determined to confirm that homozygous fIIMZ neonates had normal levels of circulating fII protein. Total RNA, harvested from fIIWT and fIIMZ livers, revealed no significant difference in fII mRNA in either fIIWT or fIIMZ mice, whereas the expected decreases in mRNA levels for both fII+/− and fIIlox/− control samples were observed (Figure 1B). Immunoblot analyses of plasma obtained from fIIWT and fIIMZ homozygous animals revealed no discernible differences in fII protein levels between genotypes (Figure 1C). Citrated plasma was also assayed for chromogenic fII activity, because it has been previously determined that both α-thrombin and meizothrombin have similar affinities for S-2238 chromogenic substrate.16 After activation of the fII in the plasma samples by ecarin, a similar level of chromogenic activity was observed between fIIWT and fIIMZ plasma samples (Figure 1D).
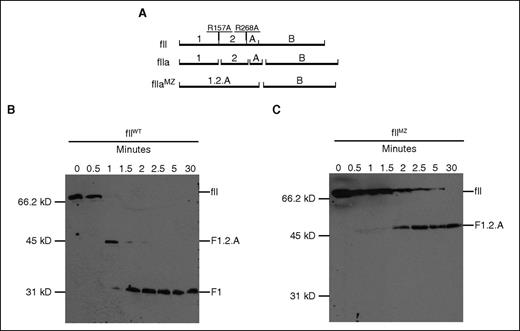
To confirm that the prothrombin generated from the fIIMZ animals was indeed limited to activation to meizothrombin, diluted plasma (1:10 with Tris-buffered saline) from fIIWT and fIIMZ animals was incubated with thromboplastin. Fibrin polymerization was blocked with the peptide Gly-Pro-Arg-Pro. Immunoblots (under reducing conditions) were performed using an antibody against an epitope at the N-terminus of the prothrombin molecule. Figure 2A details the expected fragments from fII activation in both fIIWT and fIIMZ plasma. Samples from fIIWT animals revealed a fragment consistent with activation to meizothrombin (fragment 1.2.A), which then migrated to a size consistent with fragment 1 over time (Figure 2B). However, in fIIMZ animals, no conversion beyond fIIaMZ was detected (only fragment 1.2.A was present), even after incubation for 30 minutes at 37°C (Figure 2C).
Evaluation of activation and activity of fIIMZ. (A) Schematic of prothrombin cleavage sites. An antibody was used that targets the N-terminus of prothrombin, and the immunoblot was performed under reducing conditions. Therefore, an activation event resulting in meizothrombin would result in a product including fragment 1.2 (F1.2) and the A chain (F1.2.A). Upon further cleavage to α-thrombin, fragments F1 and F2 are released from the A chain. (B-C) Activation of prothrombin was determined in both fIIWT and fIIMZ animals. Hemostatic factor activation was initiated with thromboplastin in plasma from fIIWT (B) and fIIMZ (C) animals. Aliquots were taken at specified time points and assayed via immunoblot for fII and products of fII activation. Although no remaining detectable fII or fragment 1.2.A is present in the fIIWT samples beyond 2 minutes, prothrombin from the fIIMZ animals remains at F1.2.A (representing meizothrombin) throughout the time period assayed. No further conversion/degradation was detected.
Evaluation of activation and activity of fIIMZ. (A) Schematic of prothrombin cleavage sites. An antibody was used that targets the N-terminus of prothrombin, and the immunoblot was performed under reducing conditions. Therefore, an activation event resulting in meizothrombin would result in a product including fragment 1.2 (F1.2) and the A chain (F1.2.A). Upon further cleavage to α-thrombin, fragments F1 and F2 are released from the A chain. (B-C) Activation of prothrombin was determined in both fIIWT and fIIMZ animals. Hemostatic factor activation was initiated with thromboplastin in plasma from fIIWT (B) and fIIMZ (C) animals. Aliquots were taken at specified time points and assayed via immunoblot for fII and products of fII activation. Although no remaining detectable fII or fragment 1.2.A is present in the fIIWT samples beyond 2 minutes, prothrombin from the fIIMZ animals remains at F1.2.A (representing meizothrombin) throughout the time period assayed. No further conversion/degradation was detected.
Hematologic analysis of fIIMZ animals
Complete blood count analyses on blood from fIIWT and fIIMZ mice revealed no significant difference in the white blood cell (WBC) count, hemoglobin level, or platelet count (Table 3) between genotypes. In addition, analysis of the WBC differential revealed no significant difference between fIIWT and fIIMZ animals (data not shown) in terms of leukocyte subsets. Standard coagulation function analyses revealed a significant prolongation of both the PT and aPTT for fIIMZ animals compared with fIIWT animals (Table 3). This prolongation was expected given the known diminution of fIIaMZ activity for fibrinogen. Predictably, there was no difference in the thrombin times between fIIWT and fIIMZ mice (Table 3), consistent with fIIMZ having no impact on plasma fibrinogen levels.
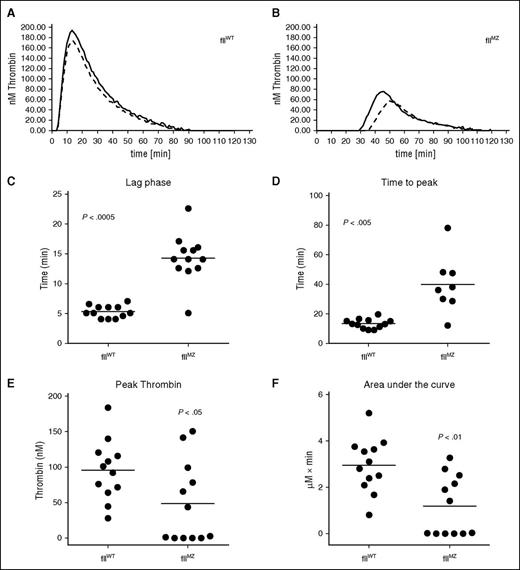
Comparative thrombin generation assays were performed on plasma from both fIIMZ and fIIWT mice (representative curves, Figure 3A-B). The fIIMZ animals had both a prolonged lag time compared with fIIWT (eg, a prolonged time to any [meizo]thrombin generation; Figure 3C) and a prolonged time to peak (meizo)thrombin production (Figure 3D). Furthermore, peak (meizo)thrombin production and area under the curve were found to be reduced in fIIMZ compared with fIIWT mice (Figure 3E-F, respectively). The Velocity Index, the rate of (meizo)thrombin generation, was also decreased in fIIMZ mice in contrast to fIIWT animals (data not shown).
Determination of the activation of fII in fIIWT and fIIMZ plasmas. To evaluate the activation potential of fII in either fIIWT or fIIMZ plasma, we used thrombin generation assays to assess fIIa/fIIaMZ generation over time. Representative thrombin generation curves from fIIWT (A) and fIIMZ (B) plasma. Time to the start of thrombin generation was significantly delayed in fIIMZ plasma (C), as well as time to peak thrombin generation (D). Peak fIIa/fIIaMZ generation was also significantly decreased in the fIIMZ animals (E). Of note, plasma from 4 fIIMZ animals did not have any appreciable (meizo)thrombin generation. Therefore, they were excluded from the time-to-peak (meizothrombin) analysis. Total fIIa/fIIaMZ generation, as represented by area under the curve, was also significantly reduced in fIIMZ plasma compared with fIIWT plasma (F).
Determination of the activation of fII in fIIWT and fIIMZ plasmas. To evaluate the activation potential of fII in either fIIWT or fIIMZ plasma, we used thrombin generation assays to assess fIIa/fIIaMZ generation over time. Representative thrombin generation curves from fIIWT (A) and fIIMZ (B) plasma. Time to the start of thrombin generation was significantly delayed in fIIMZ plasma (C), as well as time to peak thrombin generation (D). Peak fIIa/fIIaMZ generation was also significantly decreased in the fIIMZ animals (E). Of note, plasma from 4 fIIMZ animals did not have any appreciable (meizo)thrombin generation. Therefore, they were excluded from the time-to-peak (meizothrombin) analysis. Total fIIa/fIIaMZ generation, as represented by area under the curve, was also significantly reduced in fIIMZ plasma compared with fIIWT plasma (F).
To explore the contribution of fIIMZ toward activated protein C (aPC) generation, we challenged cohorts of fIIMZ and fIIWT mice with lipopolysaccharide (LPS), an inflammatory challenge known to activate the hemostatic cascade and result in aPC generation. Two hours after administration of LPS, fIIWT mice displayed the expected, and statistically significant, increase in aPC activity (supplemental Figure 2). The fIIMZ animals challenged with LPS did not display a significant difference in aPC activity compared with unchallenged fIIMZ animals. Interestingly, there was also not a statistically significant difference in aPC generation between the fIIMZ and fIIWT mice after LPS. In light of the data regarding reduced meizothrombin generation in fIIMZ animals, these data do not necessarily contradict the previously reported findings regarding activity of fIIaMZ for protein C; this finding would also be expected with diminished overall fIIaMZ activity secondary to diminished generation of fIIaMZ from fIIMZ.
Meizothrombin-induced platelet aggregation and clot retraction
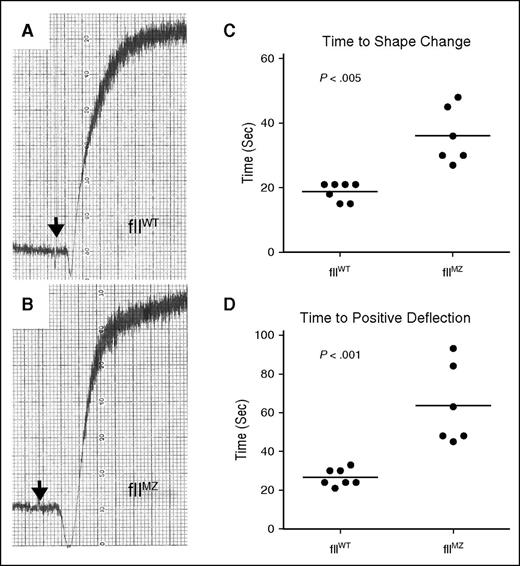
FIIa is a potent activator of platelet aggregation through activation of PARs.1 To determine the potential of fIIaMZ to activate platelets, we initiated fIIa or fIIaMZ generation in platelet-rich plasma (PRP) derived from fIIWT and fIIMZ animals. Qualitative platelet aggregation was comparable between genotypes, with similar total aggregation (Figure 4A-B; fIIWT n = 7, fIIMZ n = 7). However, there was a prolonged time to both shape change and positive deflection in fIIMZ animals (Figure 4C-D). We postulate that the prolonged time to initiation of platelet aggregation in fIIMZ plasma was a function of the extended time to (meizo)thrombin generation. More notably, these data suggest that murine fIIaMZ is capable of activating PAR-4.
Platelet aggregation in fIIMZ animals. Platelet aggregation in PRP derived from either fIIWT or fIIMZ animals in response to thromboplastin was determined. Fibrin polymerization was blocked with Gly-Pro-Arg-Pro. There was no qualitative difference between fIIWT and fIIMZ platelet aggregation (A and B, respectively). However, a modest but statistically significant difference was noted in time to shape change (as determined by negative deflection, C) and time to positive deflection (D).
Platelet aggregation in fIIMZ animals. Platelet aggregation in PRP derived from either fIIWT or fIIMZ animals in response to thromboplastin was determined. Fibrin polymerization was blocked with Gly-Pro-Arg-Pro. There was no qualitative difference between fIIWT and fIIMZ platelet aggregation (A and B, respectively). However, a modest but statistically significant difference was noted in time to shape change (as determined by negative deflection, C) and time to positive deflection (D).
Clot retraction is an important physiologic step in both hemostasis and wound repair that is dependent on fibrin polymer formation, platelet activation, and fXIII activity.25-27 To directly determine whether fIIMZ supports clot retraction, we initiated coagulation with thromboplastin in both whole blood and PRP (platelet counts adjusted to 2.5 × 105 platelets/µL) from fIIWT and fIIMZ animals. Although time to initial clot retraction was modestly delayed in fIIMZ blood and plasma samples, both fIIWT and fIIMZ displayed evidence of similar maximal clot retraction in both whole blood and PRP (supplemental Figure 3A-B,E-F). There was no qualitative difference in the size of clots by the end of the 90-minute observation period. Further, red blood cell (RBC) inclusion in thrombi was not significantly different between samples from fIIWT and fIIMZ mice (supplemental Figure 3C-D).
In vivo hemostasis and thrombosis assessment
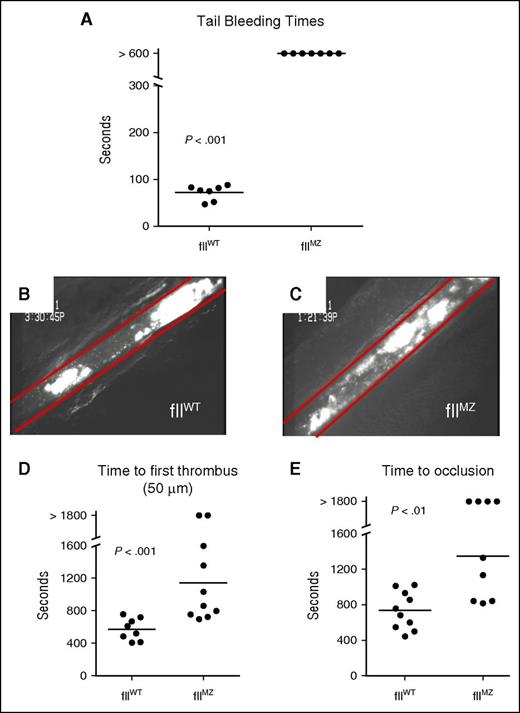
To determine the effect of limiting fII activation to meizothrombin on hemostasis in vivo, we employed a standard tail tip amputation assay. Cohorts of fIIMZ and fIIWT animals were challenged by amputation of 3 mm of the distal tail. All fIIWT mice had cessation of bleeding, with a mean time to cessation of 72 ± 6 seconds. However, all fIIMZ animals had bleeding for the entire 10-minute observation period (Figure 5A). Qualitative narrowing of the caliber of the bloodstream from the amputation site was sometimes observed in fIIMZ animals, but bleeding universally persisted throughout the observation period.
Impaired hemostasis and thrombus formation in fIIMZ animals. (A) In a standard tail bleeding time analysis, fIIWT animals all achieved hemostasis, whereas no fIIMZ mice had cessation of bleeding during the 10-minute window of evaluation. In ferric chloride mesenteric arteriole injury, fIIWT animals developed occlusive thrombi (B). However, although fIIMZ mice formed platelet aggregates, these were unstable and embolized, frequently without progression to occlusion of the vessel (C). FIIMZ animals also exhibited a prolonged time to first thrombus formation (D) and prolonged time to occlusion (E).
Impaired hemostasis and thrombus formation in fIIMZ animals. (A) In a standard tail bleeding time analysis, fIIWT animals all achieved hemostasis, whereas no fIIMZ mice had cessation of bleeding during the 10-minute window of evaluation. In ferric chloride mesenteric arteriole injury, fIIWT animals developed occlusive thrombi (B). However, although fIIMZ mice formed platelet aggregates, these were unstable and embolized, frequently without progression to occlusion of the vessel (C). FIIMZ animals also exhibited a prolonged time to first thrombus formation (D) and prolonged time to occlusion (E).
To define thrombotic potential in fIIMZ mice, we challenged fIIMZ and fIIWT mice in parallel with a ferric chloride mesenteric artery injury. Here, fluorescein-labeled platelets from donor mice of the same genotype as the challenged animals were infused before the procedure to allow real-time tracking of thrombus size and time to occlusion by intravital fluorescent microscopy. FIIWT mice demonstrated rapid formation of platelet aggregates that progressed steadily to full occlusion (Figure 5B). Although fIIMZ animals had clear evidence of platelet aggregation (Figure 5C), fIIMZ mice demonstrated a significantly increased time to first thrombus formation and prolonged time to occlusion relative to fIIWT mice, with a significant fraction of fIIMZ mice failing to form a stable occlusive thrombus during the 30-minute observation period (Figures 5D-E). In evaluation of the formation of thrombosis in the fIIMZ animals, the thrombi formed after ferric chloride injury was unstable with frequent emboli. In mice with no occlusive thrombi, a combination of delayed thrombus formation and embolization contributed to this phenotype (supplemental Video 1 [fIIWT animals] and supplemental Video 2 [fIIMZ animals]). Taken together with the platelet aggregation data, these data suggest the hypothesis that diminished fibrin polymer formation in fIIaMZ mice results in a failure to stabilize the growing platelet aggregate and a corresponding protection from arterial occlusion.
FIIMZ animals develop cardiac fibrosis
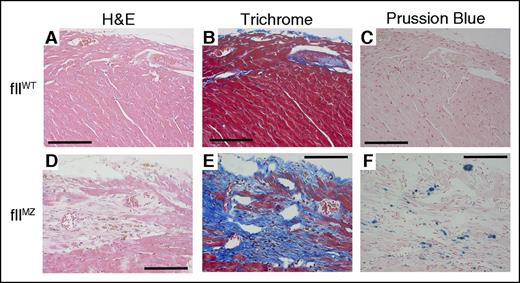
Mice with genetic deficiencies of tissue factor, fII, fVII, fX, and fXIII8,28-31 develop cardiac hemorrhage with iron deposition and subsequent fibrosis that progresses with age. To investigate whether fIIMZ predisposes adult animals to spontaneous hemorrhage, we examined organs from cohorts (10-12 weeks of age) of C57/Bl6 fIIWT and fIIMZ mice. No gross or microscopic evidence of hemorrhage was observed in the pulmonary, gastrointestinal, genitourinary, or central nervous systems of fIIWT or fIIMZ animals. As expected, fIIWT mice did not show any histologic evidence of cardiac pathologies (Figure 6A-C). In contrast, cardiac fibrosis was readily identified in both male and female fIIMZ mice (Figure 6D-E) and was most notable in the subepicardial zones. Prussian blue staining of the heart tissue revealed colocalization of iron deposits with areas of fibrosis (Figure 6F), suggesting chronic hemorrhage leading to the etiology of fibrosis in this setting.
Cardiac fibrosis in fIIMZ mice. FIIWT mice did not have any evidence of cardiac fibrosis or hemorrhage/iron deposition (A-C). However, fIIMZ animals developed evidence of cardiac fibrosis by the age of 10 weeks on both hematoxylin and eosin and Trichrome stains (D-E). At 10 weeks of age in mice, the most pronounced area of fibrosis was in the subepicardial region. Areas of fibrosis colocalize with areas of iron deposition, as shown here in Prussian blue stain (F). All scale bars represent 100 microns.
Cardiac fibrosis in fIIMZ mice. FIIWT mice did not have any evidence of cardiac fibrosis or hemorrhage/iron deposition (A-C). However, fIIMZ animals developed evidence of cardiac fibrosis by the age of 10 weeks on both hematoxylin and eosin and Trichrome stains (D-E). At 10 weeks of age in mice, the most pronounced area of fibrosis was in the subepicardial region. Areas of fibrosis colocalize with areas of iron deposition, as shown here in Prussian blue stain (F). All scale bars represent 100 microns.
In addition, cohorts of inbred C57/Bl6 fIIWT and fIIMZ mice were aged for a year before their organs were harvested. Notably, no loss of fIIMZ animals occurred during this time. Grossly, evidence of one ovarian hemorrhagic cyst and one intestinal hemorrhage were noted in the fIIMZ (n = 8) animals. There was no evidence of gross or microscopic hemorrhage in the fIIWT (n = 7) mice (supplemental Figure 4A-C). Abundant cardiac fibrosis was noted in both male and female fIIMZ animals (supplemental Figure 4D-E), whereas minimal, if any, cardiac fibrosis was noted in the fIIWT mice. Again, sites of fibrosis colocalized with sites of iron deposition (supplemental Figure 4F), likely as a result of local hemorrhage. There were no hemorrhagic findings in other organ systems.
FIIMZ mice have diminished experimental metastases
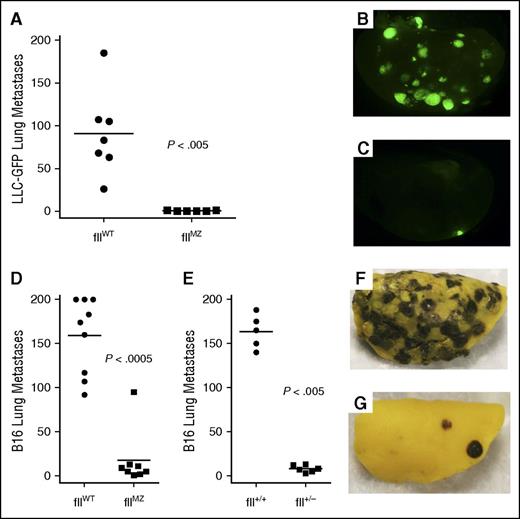
To investigate the biological consequences of fIIMZ activity in a disease context distinct from a classical hemostasis or thrombosis challenge, we examined the contribution of fIIMZ to experimental tumor metastasis. FIIa can support metastasis through tumor cell–instrinsic mechanisms involving activation of PARs,32 as well as through fibrinogen cleavage and platelet activation.23,33,34 Our hypothesis was that fIIMZ animals would demonstrate reduced numbers of metastases. Cohorts of inbred C57Bl/6-derived fIIMZ and fIIWT mice were IV injected in parallel with LLCGFP cells and the lungs harvested 14 days later. Although abundant metastases were uniformly present among the fIIWT animals, few, if any, fIIMZ animals had any evidence of metastatic foci (Figure 7A-C). This was similar to previous findings of LLC experimental lung metastases in animals with lowered levels of prothrombin.35 To expand on these findings and further compare fIIMZ with diminished levels of prothrombin, we compared experimental metastases of B16 melanoma in fIIWT, fIIMZ, and fII+/− animals. Again, fIIMZ animals demonstrated a significant protection from the development of lung metastases (Figure 7D,F-G). Evaluation of tumor metastases in mice with 50% prothrombin (fII+/−) revealed an essentially identical pattern to fIIMZ animals (Figure 7E). This suggests that even modest diminutions in thrombin generation potential, either through diminution of circulating prothrombin levels or alteration of activation, have profound effects on metastatic potential of tumor cells.
Diminished experimental tumor metastasis in fIIMZ animals. Cohorts of fIIWT and fIIMZ animals were challenged with an IV bolus of LLCGFP. Two weeks later, metastatic foci in the lung were determined. A substantial, statistically significant diminution in the number of lung metastases was noted in the fIIMZ animals compared with fIIWT mice (A). FIIWT animals had prominent metastatic foci (B), whereas many fIIMZ animals demonstrated few, if any, metastases (C). Cohorts of fIIWT and fIIMZ animals were challenged with IV bolus of B16 melanoma. Eighteen days later, metastatic foci in the lung were determined. As was seen with LLC, fIIMZ animals had significantly fewer metastatic foci when compared with fIIWT mice (D). The same pattern was found when comparing fIIWT mice with mice with 50% prothrombin (fII+/−) (E). Numerous metastatic foci were seen in fIIWT animals (F), whereas metastases were rare in fIIMZ mice (G).
Diminished experimental tumor metastasis in fIIMZ animals. Cohorts of fIIWT and fIIMZ animals were challenged with an IV bolus of LLCGFP. Two weeks later, metastatic foci in the lung were determined. A substantial, statistically significant diminution in the number of lung metastases was noted in the fIIMZ animals compared with fIIWT mice (A). FIIWT animals had prominent metastatic foci (B), whereas many fIIMZ animals demonstrated few, if any, metastases (C). Cohorts of fIIWT and fIIMZ animals were challenged with IV bolus of B16 melanoma. Eighteen days later, metastatic foci in the lung were determined. As was seen with LLC, fIIMZ animals had significantly fewer metastatic foci when compared with fIIWT mice (D). The same pattern was found when comparing fIIWT mice with mice with 50% prothrombin (fII+/−) (E). Numerous metastatic foci were seen in fIIWT animals (F), whereas metastases were rare in fIIMZ mice (G).
Discussion
Activation of thrombin follows 1 of 2 pathways, which produce functionally different intermediate species. The intermediate of the alternative prothrombin activation pathway prethrombin, lacks detectable protease activity. In contrast, meizothrombin, a short-lived activation intermediate of prothrombin,9,11 has readily detectable protease activity with notably distinct substrate specificities from α-thrombin. The pathway of thrombin activation is dictated in part by the microenvironment or cellular surface on which the prothrombinase complex is assembled. Recent data have suggested that thrombin generation on platelets primarily proceeds via prethrombin,36 whereas thrombin generation on the surface of RBCs tends to occur through meizothrombin.37 Although it has been hypothesized that the pathway of fII activation is biologically relevant, given the short half-life of fIIaMZ, the specific contribution of fIIaMZ-mediated proteolysis to hemostasis and other physiologic or pathologic processes has been difficult to assess in vivo. Here, we demonstrate for the first time the consequences of limiting the activation of endogenous fII to an activation intermediate in vivo. Mice that express fIIMZ are viable, survive well into adulthood, and have reproductive success. FIIMZ mice have partial perinatal lethality, unlike fIIlox/− animals (mice with 10% of wild-type prothrombin levels). Similar to fIIlox/− animals, fIIMZ animals that survive to weaning have an essentially normal lifespan. FIIMZ animals have significantly delayed and decreased (meizo)thrombin generation, compared with fIIWT mice. FIIMZ animals also have delayed time to occlusion after a ferric chloride arterial injury and prolonged bleeding times.
Although we did not directly measure factor XIII (fXIII) activation by fIIMZ, indirect evidence points to at least adequate activation. Clot retraction in fIIMZ plasma was normal, both in PRP and in whole blood. FXIII activity is required in the process of clot retraction and RBC retention within the thrombus.25-27 As additional evidence that fIIMZ activates fXIII, RBC retention in whole-blood thrombi after clot retraction was not significantly different between fIIWT and fIIMZ animals. Similarly, PAR-1 activation by fIIMZ has not been directly measured. However, the homozygous fIIMZ animals do not have the midgestation loss seen in PAR-1−38 and fII− animals,3,4 which would suggest that activation of PAR-1 by fIIaMZ is adequate to support successful embryonic development.
Both human and murine rfIIaMZ have decreased polymerization potential of fibrin.15,16 Our findings with in vivo fIIMZ are compatible with these results. Prolongation of the PT and aPTT are both suggestive of a decreased polymerization of fibrin (the end point of both assays). Although delayed, fIIaMZ also induces platelet aggregation qualitatively similar to α-thrombin. However, fIIMZ animals were unable to achieve hemostasis after a standard tail amputation bleeding time. This is in contrast to animals expressing a form of fibrinogen that cannot be cleaved by thrombin (FibAEK). FibAEK mice demonstrated partial cessation of bleeding in a standard tail amputation bleeding time.39 This suggests that, although inadequate fibrin polymerization contributes to abnormal hemostasis in fIIMZ mice, positive feedback for self-activation also likely participates in the failure to achieve hemostasis.
Intravital microscopy, after mesenteric arteriole ferric chloride injury, also reveals insight into the failure to form thrombi. Although fIIMZ animals formed platelet aggregates at the site of vessel injury, these aggregates were not stable. The aggregates formed after ferric chloride injury in the fIIWT controls quickly evolved into occlusive thrombi. In stark contrast, fully occlusive thrombi never formed in fIIMZ mice, rather smaller aggregates embolized from the initial thrombus in fIIMZ animals precluding occlusion. Taken together, this suggests that, in the setting of animals expressing only fIIMZ, platelet aggregation is adequate, but insufficient positive feedback to the hemostatic cascade and diminished fibrinogen cleavage lead to unstable platelet aggregates. Thus, a lack of both physiologic hemostasis and diminished thrombosis is observed in fIIMZ mice.
Previous studies have reported the effects of recombinant human and murine meizothrombin (rhfIIaMZ and rmfIIaMZ, respectively), both in vitro and in vivo.16,40-42 Similar to our current work, rmfIIaMZ demonstrated significantly diminished fibrinogen cleavage capabilities in vitro. However, in the same in vitro studies of rmfIIaMZ, increased protein C activation in the presence of thrombomodulin was observed.16 Our data suggest that the activation of protein C is not significantly different in the fIIMZ and fIIWT animals after an LPS challenge. Direct comparison between the in vitro findings and the in vivo analysis of aPC generation reported here is complicated by the overall reduced activation potential of meizothrombin in the homozygous mutant mice. Thus, we cannot further address the activity of fIIaMZ for protein C. The other significant difference in the published account of rmfIIaMZ and our current findings using knock-in animals is that rmfIIaMZ was evaluated not as a zymogen, but as a fully active enzyme. Here, we were able to explore the consequences of limiting all activation of fII to meizothrombin. Although in vitro data suggested that rhfIIaMZ would be capable of positive feedback of the hemostatic cascade, our data in murine meizothrombin suggest otherwise.12,13 Further studies will be needed to assess the in vivo activation of factors V, VIII, and XI by meizothrombin. Because (meizo)thrombin generation is significantly altered in the fIIMZ compared with fIIWT animals, this suggests meizothrombin is less effective at supporting activation of at least one of these factors. Our data challenges the hypothesis that fIIaMZ contributes to physiologic hemostasis.
Another modification of fII leading to a specificity difference, fIIWE, has diminished fibrinogen cleavage while preserving activation of protein C.43,44 Exploiting this difference in specificities, fIIaWE has been administered pharmaceutically as an anticoagulant43,45 and antiinflammatory46 agent. In addition, animals have been generated that express only the fIIWE zymogen.5 However, unlike the fIIMZ animals, mice expressing only fIIWE are not viable. The difference in viability between fIIWE and fIIMZ animals illustrates that fIIMZ does not affect physiologic hemostasis to the same degree. Further, fIIMZ animals may have an advantage in studying fII specificity alterations in physiologic or disease states.
Animals expressing fIIMZ developed cardiac fibrosis, worsening with age, similar to mice with low levels of tissue factor, fII, fVII, fX, and fXIII.8,28-31 Areas of fibrosis colocalized with areas of iron deposition, likely related to recurrent small hemorrhages. Unlike mice with low levels of fII (both fIIlox/− mice and transgenic mice expressing low levels of human prothrombin) that only develop modest cardiac fibrosis at over one year of age,8 fIIMZ mice developed substantial cardiac fibrosis by 8 to 10 weeks of age. Also unlike previous accounts of cardiac fibrosis hemostatic factor deficiencies, we did not observe a gender predisposition to the development of cardiac fibrosis. Thus, although (meizo)thrombin-induced platelet aggregation is preserved, this was not sufficient to prevent the cardiac parenchymal bleeding that leads to cardiac fibrosis. That this bleeding is limited to the heart, as opposed to bleeding patterns seen in the low tissue factor and low fVII animals, suggests tissue differences in hemostasis requirements to prevent hemorrhagic complications.47
As proof of concept that fIIMZ animals will provide a novel tool to study the biology of fII in disease, we examined the effect of limitation of the activation of fII in the setting of tumor metastasis. We found that mice with fIIMZ have dramatically reduced experimental metastasis compared with fIIWT animals. This was not necessarily a predictable result, because fibrin(ogen) and platelets both contribute to early protection of metastatic foci via protection from natural killer cells.23,33 FIIMZ retains, albeit reduced, fibrinogen cleavage potential, and essentially normal platelet activation capacity. That mice with fIIMZ had very few metastases is most likely related to the low positive feedback in generation of (meizo)thrombin combined with diminished fibrinogen cleavage and polymerization and a delay in platelet activation. However, other possible mechanisms cannot be excluded, such as diminished PAR activation in either tumor cells or nonmalignant stromal cells. Interestingly, fII+/− animals displayed a similar phenotype in tumor metastasis. FII+/− animals have ∼75% of peak thrombin generation seen in fII+/+ mice.5 Protection from metastases in fIIMZ and fII+/− underscores that tumor metastasis is quite sensitive to derangements in thrombin generation.
The current studies demonstrate that mice expressing only fIIMZ have adequate hemostasis for successful survival and reproduction and that these animals provide a unique tool to further assess the role of both prothrombin and meizothrombin in physiologic and pathologic processes. Because coagulation factors are more closely tied to nonhemostatic processes, the ability to study altered enzyme specificity in vivo will allow further elucidation of the mechanistic contribution of fII to disease. Tools, such as fIIMZ animals, will be crucial to determine how the activation and activity of fII modify disease severity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge Russell Ware for his insightful comments, and thank Gregory Adams, Whitney Miller, Malinda Frederick, Leah Rosenfeldt, Carolina Cruz, and Cheryl Rewerts for their excellent technical assistance.
This study was supported by National Institutes of Health, National Heart, Lung and Blood Institute grants K08HL105672 (E.S.M.) and R01HL102101 (D.D.W.).
Authorship
Contribution: E.S.M., M.A.S., M.J.F., J.S.P., N.L.E., C.T.E., D.D.W., and J.L.D. designed research; M.A.S., K.W.K., K.E.M., D.R.S., M.C., A.B., and E.S.M. performed research; E.S.M., M.A.S., N.L.E., C.T.E., D.D.W., and J.L.D analyzed data; and E.S.M., M.A.S., M.J.F., and J.S.P. wrote the manuscript.
Conflict-of-interest disclosure: E.S.M. has served on an advisory board for US WorldMeds and received honoraria from Baxalta for matters unrelated to this research. J.S.P. has served on an advisory board for US WorldMeds on matters unrelated to this research. The remaining declare no competing financial interests.
Correspondence: Eric S. Mullins, Division of Hematology, Cancer and Blood Diseases Institute, Cincinnati Children’s Research Foundation, MLC 7015, 3333 Burnet Ave, Cincinnati, OH 45229-3039; e-mail: eric.mullins@cchmc.org.