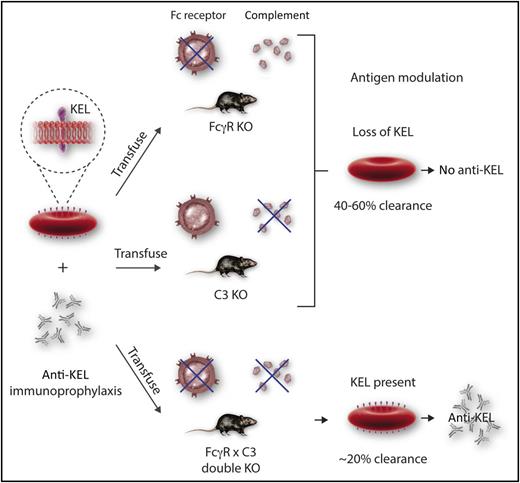
Efforts to decipher the immune mechanism underlying antibody-mediated suppression of the immune response to human KEL protein in a mouse model. IgG-mediated suppression of the immune response (AMIS) to human KEL protein occurs in the absence of FcγR or C3, but not when both FcγR and C3 are absent. Antigen modulation or clearance is associated with suppression. Professional illustration by Somersault 18:24.
Efforts to decipher the immune mechanism underlying antibody-mediated suppression of the immune response to human KEL protein in a mouse model. IgG-mediated suppression of the immune response (AMIS) to human KEL protein occurs in the absence of FcγR or C3, but not when both FcγR and C3 are absent. Antigen modulation or clearance is associated with suppression. Professional illustration by Somersault 18:24.
The authors have previously shown that mice transfused with the equivalent of 1 unit of erythrocytes expressing the human KEL glycoprotein, concurrent with passive antibody (immunoglobulin G [IgG] anti-KEL), do not make anti-KEL.2 The promise of a mouse model of antibody-mediated immune suppression is to decipher the mechanism of action of Rh immune globulin (RhIg) in the prevention of hemolytic disease of the fetus and newborn (HDFN). This therapy has been used for more than 60 years for Rh-negative mothers exposed to an Rh-positive fetus. Feto-maternal alloimmunization is characterized by the presence in a pregnant woman of alloantibodies directed against blood group antigens on red blood cells or platelets of the fetus and inherited from the father. The successful use of RhIg to prevent HDFN leaves sensitization to K antigen (Kell system) on red cells3,4 and sensitization to platelet antigen HPA-1a,5 the most common fetal complications from maternal alloimmunization. The significance of these studies lies in understanding the mechanism by which passive antibody prevents active antibody production for developing additional targets and therapies.
The study by Liu et al shows that suppression of antibody production (AMIS) in a mouse model expressing human KEL protein requires either Fcγ receptor (FcγR) or C3. Single FcγR knockout (KO) or C3 KO mice are protected (suppressed) and do not make anti-KEL. In contrast, the double-KO mice are not protected by passive immunoprophylaxis and make anti-KEL (see figure). The finding that absence of FcγR or C3 alone does not prevent AMIS was not unexpected, because previous studies have shown that AMIS occurs in mice that lack inhibitory or activating FcR, or complement, or complement receptors. These results support the contention that AMIS can result from multiple mechanisms that can replace each other functionally. The importance of the Liu et al study is that it shows the first condition in which immunoprophylaxis with passive antibody fails.
Also noteworthy is the antigen loss or modulation coincident with AMIS. Mechanistically, 40% to 60% of transfused KEL cells are rapidly cleared in single FcγR KO or C3 KO mice, while double-KO mice clear fewer cells, only about 20%. Importantly, the transfused red blood cells (RBCs) remaining in the protected (suppressed) mice have lost expression of KEL protein, as shown by flow cytometry and western blot. In double-KO mice, the majority of transfused RBCs continue to circulate and express KEL antigen (see figure).
Antigen modulation or suppression has been observed in numerous situations and results from the binding of multiple antibodies to target antigen on cells, which then lose the antigen and circulate normally. This phenomenon has been observed in vivo in humans and most often involves Kell antigens (summarized in Zimring et al6 ). Studies in mouse models demonstrated that antigen modulation on red cells required binding of antibodies that recognize different epitopes.7 C3 also plays a role,8 and some systems require activating FcRs.7
Antigen modulation has been observed for more than 30 years with antibody-based cancer therapies. With anti-CD20 (rituximab) treatment of chronic lymphocytic leukemia, neoplastic cells escape by shedding CD20.9 Antibody therapies cause internalization and capping of complexes on the surface and endocytosis of both antibody and targeted surface antigen. The major mechanism of antigen loss is mediated by FcγR, termed FcγR-mediated trogocytosis.10 Modulation of CD38 antigen on erythrocytes in patients receiving anti-CD38 therapy (daratumumab) is the most recent example familiar to transfusion medicine professionals.11 Mechanistically, the process of antigen modulation seen with monoclonal therapies may be similar to that seen here.
Relevance of the KEL mouse model to human alloimmunization remains to be determined, but one important aspect is that prevention of maternal alloimmunization to the K antigen, associated with serious and sometimes fatal fetal anemia, would be a significant advance. However, in addition to inherent differences in immune regulation in mice compared with humans, other considerations potentially impact direct translation. The human Kell polymorphism (K vs k) results from a single amino acid change; hence the number of epitopes is potentially limited in the human response. In contrast, the entire human KEL glycoprotein is the target antigen here, and the antibody response is to multiple epitopes that differ between mice and humans. Clearance mechanisms, including complement activation, crosslinking, and formation of multimeric complexes, would be predicted to potentially differ. However, the polyclonal anti-KEL response here may more closely parallel the polyclonal response to RhD antigen in humans, in which RhD-negative individuals lack the protein, and the response is directed to multiple RhD epitopes. Other variables include antigen density and copy number, glycosylation, and antigen mobility reflected by the structure of the protein in the membrane and linkage to the cytoskeleton. Kell is a single-pass glycosylated protein, and Rh is a nonglycosylated multipass protein linked to the cytoskeleton. Complement activation, antigen shedding or loss, and antigen processing may be antigen dependent.
Importantly, the Liu et al study has the potential to add insight for understanding intrinsic regulation of the human immune response. Specifically, biological systems, including the immune system, are self-regulating. Because introduction of antigen elicits a response, its clearance causes the response to cease. Mechanistic investigations of antibody-mediated immune suppression will potentially result in a better understanding of the regulatory mechanism of clearance and downregulation of the immune response. (In support, double-KO mice generate statistically significantly more anti-KEL.) Insights may make it possible to control unwanted responses, including those associated with maternal and transfusion-associated alloimmunization.
It has long been recognized that passively transferring antibody or effector T cells from an immunized individual to naïve recipients prevents activation of naïve B and T cells and, consequently, the naïve lymphocytes do not respond to the antigen. It has been assumed that crosslinking of the antigen receptor on B cells to FcγRII on the same B cell inhibits activation of naïve B cells and explains antibody-mediated immune suppression seen in prevention of the RhD response in Rh-negative mothers to their Rh-positive children. These studies pave the way for confirmation, or not, of this supposition and will lead to understanding the process.
Conflict-of-interest disclosure: The author declares no competing financial interests.