Key Points
Despite immunoprophylaxis with anti-KEL sera, mice lacking both Fcγ receptors and C3 become alloimmunized to transfused KEL RBCs.
Antigen modulation may be important in the mechanism of action of immunoprophylaxis therapies against RBC antigens.
Red blood cell (RBC) alloimmunization is a serious complication of transfusion or pregnancy. Despite the widespread use of Rh immune globulin to prevent pregnancy associated anti-D alloimmunization, its mechanism of action remains elusive. We have previously described a murine model in which immunoprophylaxis with polyclonal anti-KEL sera prevents alloimmunization in wild-type recipients transfused with transgenic murine RBCs expressing the human KEL glycoprotein. To investigate the mechanism of action, we have now evaluated the outcome of immunoprophylaxis treatment in mice lacking Fcγ receptors (FcγRs), complement (C3), both, or none. Whereas polyclonal anti-KEL sera completely prevented alloimmunization in wild-type and single-knockout (KO) mice lacking FcγRs or C3, double-KO mice lacking both FcγRs and C3 became alloimmunized despite immunoprophylaxis. Rapid clearance of essentially all transfused RBCs with detectable KEL glycoprotein antigen occurred within 24 hours in wild-type and single-KO recipients treated with immunoprophylaxis, with the transfused RBCs remaining in circulation having minimal KEL glycoprotein antigen detectable by flow cytometry or western blot. In contrast, transfused RBCs with the KEL glycoprotein antigen fully intact continued to circulate for days in double-KO mice despite treatment with immunoprophylaxis. Further, in vitro phagocytosis assays showed no consumption of opsonized murine RBCs by double-KO splenocytes. Taken in combination, our data suggest that modulation of the KEL antigen (and potentially RBC clearance) by redundant recipient pathways involving both FcγRs and C3 may be critical to the mechanism of action of polyclonal anti-KEL immunoprophylaxis. These findings could have implications for the development of immunoprophylaxis programs in humans.
Introduction
Red blood cell (RBC) alloimmunization, or the formation of antibodies to non–self-RBC antigens after exposure during transfusion or pregnancy, is a clinically significant problem.1 Alloantibodies limit the availability of compatible RBCs and increase the risk of hemolytic transfusion reactions in a transfusion setting. Alloantibodies generated during pregnancy may lead to anemia of the current fetus and may also impact future pregnancies.2 The vast majority of alloimmunization prevention relies on antigen avoidance. However, such avoidance is not practical in many situations and, at present, is essentially impossible in the setting of pregnancy. Reported rates of alloantibody-associated deaths reported to the US Food and Drug Administration have not significantly changed in recent years,3 despite guidelines endorsing more restrictive transfusion practices. Further, although phenotypic antigen matching has decreased alloimmunization in some settings,4 it has not eliminated alloimmunization.5
To date, Rh immune globulin (RhIg) is the only therapy available to effectively prevent alloimmunization. RhIg is primarily used during pregnancy, and its impact is limited to mitigating alloimmunization to only a single antigen (RhD). Nonetheless, RhIg has had an incredible impact on the prevalence of hemolytic disease of the fetus and newborn due to anti-Rh(D), decreasing the incidence of alloimmunization during an at-risk pregnancy from 16% to <0.1%6 and the associated complications of such antibody development. Although in widespread use for >50 years, its mechanism of action remains poorly understood.7,8 Further, differences in fucosylation patterns of RhIg by manufacturer have recently been described,9 with these differences theoretically being capable of impacting efficacy.10 The desire to understand the mechanism of action of RhIg is multifold: (1) a more standard RhIg product9 could potentially be developed, be it a polyclonal anti-D preparation from volunteer donors or a monoclonal anti-D or monoclonal cocktail developed in a laboratory11,12 ; and (2) therapeutics against RBC antigens other than RhD or against platelet-specific antigens could potentially be established for clinically significant antigens encountered in the settings of pregnancy and transfusion.
Given difficulties in developing a murine model of RhD alloimmunization,13 models of antibody prophylaxis to transfused RBCs have to date been limited to xenoantigens (sheep RBCs transfused into mice),14,15 to model antigens expressed on murine RBCs (such as HOD [hen egg lysozyme, ovalbumin, human duffyb]),16 and to human antigens expressed on murine RBCs (such as the KEL glycoprotein).17 Findings in these models argue against the importance of RBC clearance or “epitope masking” in the mechanism of action of antibody-mediated immune suppression.16 Further, findings in these models argue against the sole importance of inhibitory or activating Fcγ receptors (FcγRs)18 or complement or complement receptors.19 Thus, the mechanism of action through which monoclonal or polyclonal antibodies prevent alloimmunization remains elusive.7
We have recently described that immunoprophylaxis using polyclonal antibodies to murine RBCs expressing the human KEL glycoprotein prevents alloimmunization to transfused KEL RBCs.17 We have also observed that RBCs expressing high levels of KEL transfused into mice treated with anti-KEL sera are rapidly cleared despite a lack of FcγRs or C3, albeit with slightly altered kinetics compared with wild-type mice.20 Those studies also suggested, for the first time, that FcγRs and complement may work in tandem to modulate a blood group antigen. Taking our past data in combination with immunoprophylaxis data in other models, we now hypothesize that the mechanism of action of anti-KEL immunoprophylaxis may involve FcγR or C3 modulation of the KEL antigen, and we undertook the current studies in mice lacking FcγRs, C3, or both (double knockout [KO]) to test that hypothesis.
Methods
Mice
C57BL/6 mice were purchased from Taconic (Hudson, NY). Complement C3 KO, common FcγR chain KO (Fcer1g), and MuMT mice were purchased from The Jackson Laboratory (Bar Harbor, ME); mice lacking both FcγRs and C3 were bred by our laboratory. Transgenic mice expressing the entire human KEL glycoprotein17 were generated and bred by our laboratory. The mice used for these experiments have been previously described as “KEL2B,”21 because they express the KEL2 antigen in addition to the Jsb antigen, the Kpb antigen, and many other antigens in the KEL family on their RBCs. In this article, they are referred to as “KEL” mice for simplicity, given that the protein being studied includes the entire human KEL glycoprotein. All animals were housed in Yale University’s animal facilities.
Polyclonal antibody generation and immunization
Antiserum against the KEL glycoprotein was generated as previously described17 by transfusing transgenic RBCs into C57BL/6 recipients pretreated with an intraperitoneal injection of 100 μg high-molecular-weight poly(I:C) (InvivoGen, San Diego, CA) a total of 3 times, separated by 2 weeks between each injection. Pooled sera collected 2 to 4 weeks after the final transfusion was tested for KEL binding ability by flow crossmatch with KEL2 or control C57BL/6 RBCs as targets. Following dose-titration experiments, recipient mice were passively transferred with 20 μl anti-KEL sera IV 2 to 4 hours prior to transfusion.
Blood collection, fluorescent labeling, and transfusion
Donor KEL or wild-type C57BL/6 RBCs were collected into anticoagulant preservative solution (CPDA, citrate phosphorus dextrose adenine, Jorgensen Labs, Henry Schein, Melville, NY), leukoreduced over a syringe filter (Pall Corporation, Port Washington, NY), and washed to remove residual citrate. Prior to transfusion, RBCs were labeled with chloromethylbenzamido 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (CM-DiI) or 3,3′-dihexadecyloxacarbocyanine perchlorate (DiO) according to the manufacturer’s instructions (Molecular Probes, Eugene, OR) and as previously described.20 Recipient mice were transfused via lateral tail vein with the equivalent of 1 U KEL RBCs and a similar amount of control RBCs. This amount of RBCs was selected for these experiments, given the robust anti-KEL response known to occur following transfusion.22 Survival of the transfused RBCs was determined by comparing the ratio of circulating KEL to control RBCs in recipients at select time points after transfusion.
Flow cytometry and western blots
To evaluate the active immune response to mice after transfusion, serum was collected at multiple time points and the anti-KEL immunoglobulin M (IgM) or IgG responses were measured using a flow cytometric crossmatch assay with antigen-positive (KEL) or antigen-negative (C57BL/6) RBC targets. Secondary antibodies included goat anti-mouse immunoglobulins (BD Biosciences, San Jose, CA), goat anti–mouse IgG (Jackson ImmunoResearch, West Grove, PA) or F(ab′)2 fragment IgM μ chain specific (Jackson ImmunoResearch). The antigen-specific response (adjusted mean fluorescence intensity [MFI]) was determined by subtracting the signal of serum with antigen-negative C57BL/6 RBCs from that of serum with antigen-positive RBCs.
Transfused RBCs were analyzed for the presence of bound anti-KEL passively administered antibody by a direct antiglobulin test, where anti–mouse IgG was reacted directly with fluorescently labeled RBCs recovered from the transfused animal. Levels of KEL antigen on these same RBCs were evaluated by incubating them with polyclonal anti-KEL sera, followed by anti–mouse IgG. Transfused RBCs were analyzed for binding of all forms of C3 using biotinylated rat anti–mouse complement component C3 (clone RmC11H9; Cedarlane, Burlington, ON, Canada), followed by streptavidin-conjugated to allophycocyanin secondary (BD Biosciences). Samples were analyzed on a BD FACSCalibur, a BD LSR-II, or a Miltenyi MACSQuant analyzer.
For western blots, RBCs from transfused recipients were lysed in hypotonic sodium phosphate buffer and membranes were run under reducing conditions.21 The KEL glycoprotein was detected using a mouse monoclonal antibody against an epitope of this glycoprotein (clone MM0435-12X3; Novus Biologicals, Littleton, CO), with goat anti–mouse IgG1 HRP (Bethyl Laboratories, Montgomery, TX) being used as the secondary antibody. Actin was used as a loading control. Bands were detected using the Immobilon Western Chemiluminescent HRP Substrate (Millipore, Darmstadt, Germany).
In vitro phagocytosis assay
Splenocytes from the described strains of mice were processed in media and incubated at 37°C for 2 hours. KEL RBCs that had been preincubated with anti-KEL sera for 60 minutes were added to the adherent monocytes/macrophages, and the mixture was incubated for 15 to 30 minutes. Following staining, phagocytosis was evaluated by light microscopy by a pathologist blinded to the conditions of the experiments.
Statistics
All statistical analysis was performed using GraphPad Prism software (San Diego, CA). A Mann-Whitney U test was used to determine significant differences between 2 groups, with 1-way ANOVA with Tukey’s multiple comparisons test or 2-way ANOVA completed in relevant experiments. Error bars represent 1 standard deviation (SD), and significance was determined by a P value < .05.
Study approval
All procedures and protocols were approved by Yale University’s institutional care and use committee.
Results
Anti-KEL immunoprophylaxis does not prevent alloimmunization in double-KO recipients lacking both FcγRs and C3
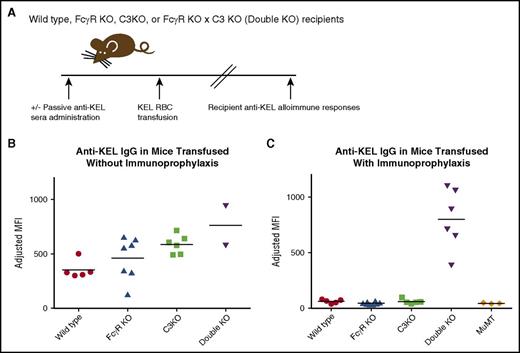
We have previously described the efficacy of passively administered anti-KEL sera at preventing alloimmunization to transfused KEL RBCs in wild type recipients.17 As part of the investigation into possible mechanism(s) of action of immunoprophylaxis, recipients genetically lacking FcγRs, C3, or both FcγRs and C3 (double KO) were studied (Figure 1A depicts the experimental design). Given studies by others demonstrating that the presence of complement may impact responses to transfused RBCs in general,19 initial studies focused on the alloimmune response of these recipients to transfused RBCs without any prior immunoprophylaxis therapy. The equivalent of 1 human unit of leukoreduced KEL RBCs was transfused via the lateral tail vein into wild-type, FcγR KO mice, C3 KO mice, and FcγR × C3 double-KO mice, and serum was collected at multiple time points after transfusion. All recipients generated robust levels of anti-KEL IgG (Figure 1B shows data from 2 combined experiments, n = 20 mice total, 21-28 days after transfusion). By conducting a 1-way ANOVA of the data in Figure 1B, we found that the double-KO mice generated statistically significantly more anti-KEL than the other groups (P < .05).
Immunoprophylaxis with anti-KEL sera prevents alloimmunization in wild-type, FcγR KO, and C3 KO mice, but not in FcγR KO × C3 KO (double-KO) mice. (A) General experimental design. Recipients were passively immunized with anti-KEL sera in some experiments, followed by transfusion of murine RBCs expressing the human KEL glycoprotein. Alloimmune responses were measured in serum after transfusion. (B) Total anti-KEL IgG measured in the serum of recipients on day 28 posttransfusion. (C) Total anti-KEL IgG measured in the serum of recipients treated with anti-KEL immunoprophylaxis prior to RBC exposure; P < .05 by 1-way ANOVA between double-KO and all other recipients. These data are a compilation of 2 independent experiments with 2 or 3 mice per group per experiment.
Immunoprophylaxis with anti-KEL sera prevents alloimmunization in wild-type, FcγR KO, and C3 KO mice, but not in FcγR KO × C3 KO (double-KO) mice. (A) General experimental design. Recipients were passively immunized with anti-KEL sera in some experiments, followed by transfusion of murine RBCs expressing the human KEL glycoprotein. Alloimmune responses were measured in serum after transfusion. (B) Total anti-KEL IgG measured in the serum of recipients on day 28 posttransfusion. (C) Total anti-KEL IgG measured in the serum of recipients treated with anti-KEL immunoprophylaxis prior to RBC exposure; P < .05 by 1-way ANOVA between double-KO and all other recipients. These data are a compilation of 2 independent experiments with 2 or 3 mice per group per experiment.
Having established an ability of all the recipients to form anti-KEL RBC alloantibodies, experiments were then completed in the presence of immunoprophylaxis against the transfused RBCs. Anti-KEL sera (with all IgG subtypes but no detectable IgM)17 was passively transferred to recipients prior to the transfusion of KEL RBCs, and recipient alloantibody responses were measured by flow cytometric crossmatch; agammaglobulinemic MuMT recipients were included as controls for detection of passively administered antibody in the absence of active anti-KEL formation (anti-KEL MFI 43, SD 3). Whereas immunoprophylaxis with passive anti-KEL sera prevented active humoral immune responses in wild-type (anti-KEL MFI 61.2, SD 18.4), FcγR KO (MFI 44.3, SD 11.9), and C3 KO (MFI 60.2, SD 21.3) recipients, this therapy was ineffective at preventing anti-KEL responses in double-KO recipients lacking both FcγRs and C3 (MFI 800, SD 271.5; P < .05 compared with other groups) (Figure 1C).
KEL RBCs have altered clearance patterns in the presence of immunoprophylaxis in double-KO mice lacking both FcγRs and C3 compared with wild-type and single-KO mice
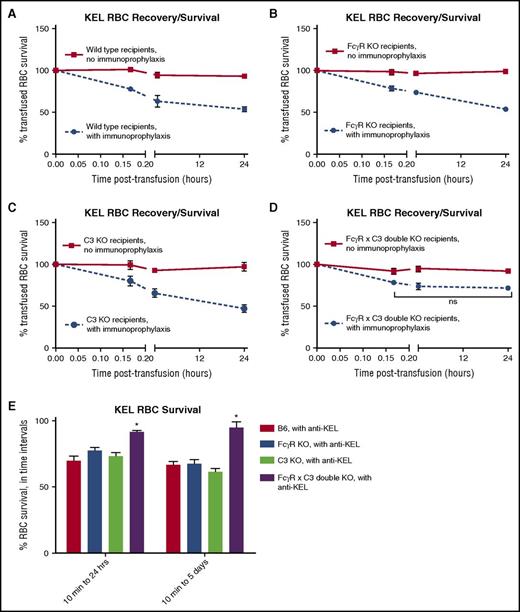
Having established that the combination of FcγRs and C3 was important to the efficacy of immunoprophylaxis with anti-KEL sera, experiments were then designed to investigate why this might be the case. Clearance patterns of transfused RBCs have been shown to impact the immunogenicity in other murine models, and thus these patterns were initially selected for study. KEL RBCs were labeled with a lipophilic dye, and wild-type RBCs were labeled with a different lipophilic dye. A mixture of these RBCs was transfused, with posttransfusion recovery and clearance measured on serial samples of peripheral blood by analyzing the ratios of KEL and wild-type lipophilic dye fluorescence using flow cytometry. Consistent with our prior observations,17 anti-KEL prophylaxis resulted in the rapid clearance of 40% to 60% of transfused, lipophilically labeled KEL RBCs within 24 hours after transfusion in wild-type mice (Figure 2A). Likewise, a similar amount of KEL RBC clearance was observed in single-KO mice lacking FcγRs as well as in single-KO mice lacking C3 (Figure 2B-C). In contrast, although ∼20% of transfused KEL RBCs appeared to clear in the immediate posttransfusion period in double-KO mice treated with immunoprophylaxis, there was essentially no KEL RBC clearance noted in the 10-minute to 24-hour posttransfusion period in these recipients (Figure 2D). Statistically significant differences were noted in the degree of RBC clearance between the double-KO mice and wild-type mice treated with immunoprophylaxis at 1 hour (P < .001) and 24 hours (P < .0001) after transfusion, with statistically significant differences also noted in the degree of RBC clearance between the double-KO mice and single-KO mice treated with immunoprophylaxis 24 hours after transfusion (P < .0001). Similar clearance patterns were observed in 2 or 3 experiments, with 3 to 5 mice per group per experiment. To more closely evaluate KEL RBC survival in recipients treated with immunoprophylaxis, the percentage of KEL RBCs remaining between 10 minutes and 24 hours after transfusion was calculated (Figure 2E, left bars), as was the percentage remaining between 10 minutes and 5 days after transfusion (Figure 2E, right bars; P < .05 by 2-way ANOVA between double-KO and other recipients in both of these evaluated time intervals).
Immunoprophylaxis with anti-KEL sera leads to less RBC clearance in FcγR KO × C3 KO (double-KO) recipients compared with wild-type, FcγR KO, or C3 KO recipients. Posttransfusion clearance curves of KEL RBCs in (A) wild-type, (B) FcγR KO, (C) C3 KO, and (D) FcγR KO × C3 KO (double-KO) recipients, in the absence (solid line) or presence (dashed line) of immunoprophylaxis, to 24 hours. These data are representative of 2 or 3 independent experiments with 3 to 5 mice per group per experiment; error bars indicate SD between individual mice. (E) Comparison of percentage of KEL RBCs remaining between 10 minutes and 24 hours after transfusion (left) or 10 minutes and 5 days after transfusion (right) in the presence of anti-KEL immunoprophylaxis; *P < .05 by 2-way ANOVA between double-KO and all other recipients.
Immunoprophylaxis with anti-KEL sera leads to less RBC clearance in FcγR KO × C3 KO (double-KO) recipients compared with wild-type, FcγR KO, or C3 KO recipients. Posttransfusion clearance curves of KEL RBCs in (A) wild-type, (B) FcγR KO, (C) C3 KO, and (D) FcγR KO × C3 KO (double-KO) recipients, in the absence (solid line) or presence (dashed line) of immunoprophylaxis, to 24 hours. These data are representative of 2 or 3 independent experiments with 3 to 5 mice per group per experiment; error bars indicate SD between individual mice. (E) Comparison of percentage of KEL RBCs remaining between 10 minutes and 24 hours after transfusion (left) or 10 minutes and 5 days after transfusion (right) in the presence of anti-KEL immunoprophylaxis; *P < .05 by 2-way ANOVA between double-KO and all other recipients.
No in vitro phagocytosis of opsonized KEL RBCs was observed in splenocytes from double-KO mice
Monocyte monolayer assays are completed by reference laboratories in the field of transfusion medicine to predict the clinical significance of an antibody using in vitro testing.23 A murine version of this assay was developed to investigate the consumption of incompatible RBCs in vitro and to supplement the data obtained in vivo. Initially, this assay was optimized using wild-type mouse splenocytes incubated with sheep RBCs that had been incubated with anti–sheep antibody. Consumption of sheep RBCs by phagocytic cells was visualized by light microscopy after 15 minutes of incubation (supplemental Figure 1A, available on the Blood Web site), with no phagocytosis observed in the absence of anti–sheep RBC antibody at low power and after evaluating 30 high-power fields (×1000) (supplemental Figure 1B).
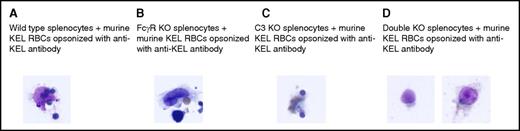
Following assay optimization with sheep RBCs, studies were completed using murine RBCs. Splenocytes from wild-type mice, FcγR KO mice, C3 KO mice, or double-KO mice lacking both C3 and FcγRs were incubated with transgenic murine KEL RBCs that had been incubated with anti-KEL sera. Consumption of murine RBCs by phagocytes from wild-type, FcγR KO, and C3 KO mice was evident after 15 minutes of incubation, with 2 or 3 phagocytes per 30 high-power fields observed to consume murine RBCs (Figure 3A-C). This consumption was dependent on the presence of anti-KEL, with no phagocytosis observed after up to 30 minutes of incubation in wells with splenocytes and RBCs alone. In contrast, no phagocytosis of opsonized murine RBCs was observed to occur by the splenocytes of double-KO mice after 15 minutes (Figure 3D) or 30 minutes of incubation.
In vitro phagocytosis of antibody-coated KEL RBCs is observed in wild-type, FcγR KO, and C3 KO, but not FcγR KO × C3 KO (double-KO), splenocytes. Opsonized murine KEL RBCs were incubated in vitro for 15 minutes with splenocytes from (A) wild-type (C57BL/6) mice, (B) FcγR KO mice, (C) C3 KO mice, or (D) double-KO mice lacking FcγR and C3. Scanning was initially completed at low power, and then 30 high-power fields were evaluated in duplicate wells. The microscope used was an Olympus BX40, with an Olympus PLAN 100× objective and a numerical aperture of 1.25 under oil emersion. Images were captured using a SPOT Insight CCD camera (model 14.2) using SPOT basic software (version 4.7).
In vitro phagocytosis of antibody-coated KEL RBCs is observed in wild-type, FcγR KO, and C3 KO, but not FcγR KO × C3 KO (double-KO), splenocytes. Opsonized murine KEL RBCs were incubated in vitro for 15 minutes with splenocytes from (A) wild-type (C57BL/6) mice, (B) FcγR KO mice, (C) C3 KO mice, or (D) double-KO mice lacking FcγR and C3. Scanning was initially completed at low power, and then 30 high-power fields were evaluated in duplicate wells. The microscope used was an Olympus BX40, with an Olympus PLAN 100× objective and a numerical aperture of 1.25 under oil emersion. Images were captured using a SPOT Insight CCD camera (model 14.2) using SPOT basic software (version 4.7).
Modulation of the KEL antigen on transfused RBCs in the presence of immunoprophylaxis requires FcγRs or C3
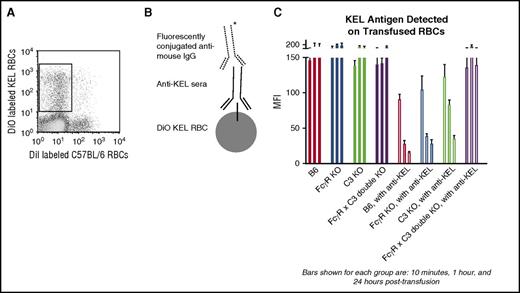
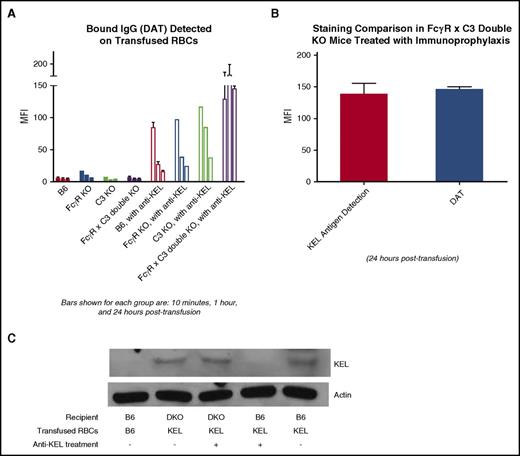
Modulation of the KEL antigen on transfused RBCs, to the point of the antigen being largely undetectable by direct flow cytometric methods or intracellular staining, has previously been observed by our laboratory in wild-type mice transfused with KEL RBCs in the presence of anti-KEL immunoprophylaxis.17 However, the importance of antigen modulation on the efficacy of anti-KEL immunoprophylaxis has remained unanswered to date. To investigate this issue, wild-type, FcγR KO, C3 KO, and FcγR × C3 double-KO mice were treated with or without anti-KEL immunoprophylaxis and then transfused with lipophilically labeled KEL RBCs. These labeled RBCs were recovered from the peripheral circulation of the mice at early (10 minutes, 1 hour) and later (24 hours) time points after transfusion (Figure 4A) and stained with anti-KEL sera followed by fluorescently conjugated anti–mouse immunoglobulins (Figure 4B shows the staining schematic). As previously described,21 the KEL antigen is stable on transfused RBCs in the absence of immunoprophylaxis (Figure 4C, left side). Whereas the KEL antigen rapidly became almost undetectable on KEL RBCs transfused into wild-type, FcγR KO, and C3KO recipients pretreated with immunoprophylaxis, the antigen remained detectable and largely unchanged for 24 hours after transfusion in double-KO mice lacking FcγRs and C3 pretreated with immunoprophylaxis (Figure 4C, right side; P < .05 at 1 hour and 24 hours after transfusion by 2-way ANOVA between double-KO and other recipients treated with immunoprophylaxis).
Modulation of the KEL antigen on transfused RBCs in the presence of immunoprophylaxis requires FcγRs or C3. KEL RBCs labeled with the lipophilic dye DiO were recovered from recipients transfused in the absence or presence of anti-KEL immunoprophylaxis 10 minutes, 1 hour, and 24 hours after transfusion (A) and incubated with anti-KEL sera followed by fluorescently conjugated anti–mouse IgG (schematic shown in B). (C) Detection of the KEL antigen on recovered lipophilically labeled KEL RBCs in wild-type, FcγR KO, C3R KO, and FcγR × C3 KO (double-KO) recipients, transfused in the presence or absence of anti-KEL immunoprophylaxis (P < .05 at 1 hour and 24 hours after transfusion by 2-way ANOVA between double-KO and other recipients treated with immunoprophylaxis). These data are representative of 2 or 3 independent experiments with 3 mice per group per experiment; error bars indicate SD between mice.
Modulation of the KEL antigen on transfused RBCs in the presence of immunoprophylaxis requires FcγRs or C3. KEL RBCs labeled with the lipophilic dye DiO were recovered from recipients transfused in the absence or presence of anti-KEL immunoprophylaxis 10 minutes, 1 hour, and 24 hours after transfusion (A) and incubated with anti-KEL sera followed by fluorescently conjugated anti–mouse IgG (schematic shown in B). (C) Detection of the KEL antigen on recovered lipophilically labeled KEL RBCs in wild-type, FcγR KO, C3R KO, and FcγR × C3 KO (double-KO) recipients, transfused in the presence or absence of anti-KEL immunoprophylaxis (P < .05 at 1 hour and 24 hours after transfusion by 2-way ANOVA between double-KO and other recipients treated with immunoprophylaxis). These data are representative of 2 or 3 independent experiments with 3 mice per group per experiment; error bars indicate SD between mice.
Near-complete modulation of the KEL antigen in wild-type, but not FcγR KO ×C3 KO recipients, is corroborated by direct antiglobulin testing and western blot
The lack of detectable antigen on recovered KEL RBCs in wild-type, FcγR KO, and C3KO mice was unlikely to be due to “masking” of the antigen by the passively infused anti-KEL immunoprophylaxis, as staining of the DiO-positive RBCs in these mice with fluorescently conjugated anti–mouse immunoglobulins alone showed little residual anti-KEL bound within 24 hours after transfusion (Figure 5A, right side; P < .05 at 1 hour and 24 hours after transfusion by 2-way ANOVA between double-KO and other recipients treated with immunoprophylaxis). In comparison, such staining of the recovered KEL RBCs in double-KO mice lacking both FcγRs and C3 was equally as positive to the staining of these KELs with anti-KEL sera plus fluorescently conjugated immunoglobulins (Figure 5B). These data are consistent with essentially all epitopes on the KEL glycoprotein being bound following the in vivo administration of anti-KEL immunoprophylaxis, with presumed intact KEL glycoprotein antigen and persistent detection of bound anti-KEL antibody at 24 hours after transfusion in DKO recipients.
Near-complete removal of the KEL glycoprotein antigen on RBCs occurs in wild-type, but not FcγR KO × C3 KO (double-KO), recipients treated with anti-KEL immunoprophylaxis. (A) KEL RBCs labeled with the lipophilic dye DiO were recovered from recipients transfused in the absence or presence of anti-KEL immunoprophylaxis 10 minutes, 1 hour, and 24 hours after transfusion and incubated directly with fluorescently conjugated anti–mouse IgG (P < .05 at 1 hour and 24 hours after transfusion by 2-way ANOVA between double-KO and other recipients treated with immunoprophylaxis). (B) Side-by-side comparison of the KEL antigen detection signal versus the signal of RBCs incubated directly with fluorescently conjugated antibody 24 hours after transfusion, in FcγR × C3 (double-KO) recipients treated with anti-KEL immunoprophylaxis. (C) Western blot data of membrane preps from RBCs recovered from wild-type (B6) or FcγR × C3 (double-KO) mice 24 hours after transfusion, in the absence or presence of treatment with anti-KEL immunoprophylaxis. The data in A and B are representative of 2 to 3 independent experiments with 3 mice per group per experiment; error bars indicate SD. The data in C are representative of 2 independent experiments with 2 mice per group per experiment.
Near-complete removal of the KEL glycoprotein antigen on RBCs occurs in wild-type, but not FcγR KO × C3 KO (double-KO), recipients treated with anti-KEL immunoprophylaxis. (A) KEL RBCs labeled with the lipophilic dye DiO were recovered from recipients transfused in the absence or presence of anti-KEL immunoprophylaxis 10 minutes, 1 hour, and 24 hours after transfusion and incubated directly with fluorescently conjugated anti–mouse IgG (P < .05 at 1 hour and 24 hours after transfusion by 2-way ANOVA between double-KO and other recipients treated with immunoprophylaxis). (B) Side-by-side comparison of the KEL antigen detection signal versus the signal of RBCs incubated directly with fluorescently conjugated antibody 24 hours after transfusion, in FcγR × C3 (double-KO) recipients treated with anti-KEL immunoprophylaxis. (C) Western blot data of membrane preps from RBCs recovered from wild-type (B6) or FcγR × C3 (double-KO) mice 24 hours after transfusion, in the absence or presence of treatment with anti-KEL immunoprophylaxis. The data in A and B are representative of 2 to 3 independent experiments with 3 mice per group per experiment; error bars indicate SD. The data in C are representative of 2 independent experiments with 2 mice per group per experiment.
To further investigate the status of the KEL glycoprotein on transfused KEL RBCs in the presence or absence of anti-KEL immunoprophylaxis, western blots were completed on membranes generated from RBCs recovered from recipients 24 hours after transfusion. For initial optimization of this assay, RBC membranes from wild-type B6 or KEL donors were run on a gel under reducing conditions and probed with a monoclonal antibody against an extracellular epitope of the KEL glycoprotein; a KEL band was identified at ∼83 kDa in KEL, but not B6, RBC membranes. Essentially no KEL signal was detectable on the RBC membranes from B6 recipients treated with anti-KEL immunoprophylaxis prior to KEL RBC transfusion (Figure 5C, second lane from right). In contrast, a KEL signal was detected on the RBC membranes from B6 recipients transfused with KEL RBCs (Figure 5C, rightmost lane). Further, a KEL signal was detected on the RBC membranes from FcγR × C3 double-KO mice transfused with KEL RBCs regardless of whether the recipients were pretreated with anti-KEL immunoprophylaxis or not (Figure 5C, second and third lanes from the left, respectively). Taken in combination with the flow cytometric data, these data are consistent with anti-KEL immunoprophylaxis resulting in essentially complete antigen modulation of the KEL glycoprotein from transfused RBCs in B6, but not FcγR × C3 double-KO, mice.
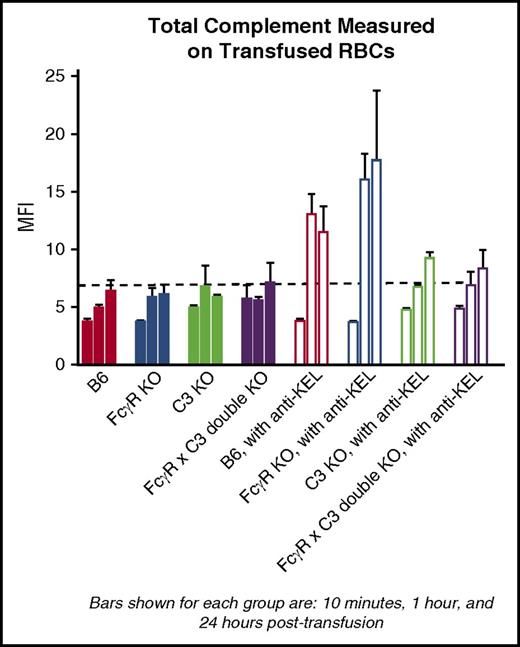
C3 binds to transfused KEL RBCs in wild-type and FcγR KO mice in the presence of immunoprophylaxis
The data presented thus far, in combination with data previously generated in our laboratory in a different KEL model in which the KEL glycoprotein is present at a higher density,20 suggest that complement plays a role (albeit presumably a redundant role) in the clearance patterns and antigen modulation of KEL RBCs in the presence of immunoprophylaxis with polyclonal anti-KEL sera. To investigate this role further, transfused RBCs were recovered from animals treated or not treated with anti-KEL immunoprophylaxis and stained with a reagent that recognizes all forms of C3 at early (10 minutes, 1 hour) and later (24 hours) time points after transfusion. Total C3 was detectable on transfused RBCs in wild-type and FcγR KO mice by 1 hour after transfusion and remained present at 24 hours after transfusion (Figure 6 shows total C3 at 10 minutes, 1 hour, and 24 hours after transfusion). In contrast, total C3 was essentially undetectable above background in C3 KO and in FcγR KO × C3 KO (double-KO) recipients.
Total C3 detected on transfused KEL RBCs in wild-type and FcγR KO mice. Posttransfusion measurements of total C3 on lipophilically labeled KEL RBCs in different strains of recipients, transfused in the absence or presence of anti-KEL immunoprophylaxis. These data, shown at 10 minutes, 1 hour, and 24 hours after transfusion, are representative of 2 independent experiments with 3 mice per group per experiment; error bars indicate SD.
Total C3 detected on transfused KEL RBCs in wild-type and FcγR KO mice. Posttransfusion measurements of total C3 on lipophilically labeled KEL RBCs in different strains of recipients, transfused in the absence or presence of anti-KEL immunoprophylaxis. These data, shown at 10 minutes, 1 hour, and 24 hours after transfusion, are representative of 2 independent experiments with 3 mice per group per experiment; error bars indicate SD.
Discussion
In this article, we describe the first set of conditions discovered to date in which immunoprophylaxis fails to prevent alloimmunization, despite antibody engagement of an authentic human blood group protein expressed on murine RBCs. Passive administration of polyclonal anti-KEL sera containing all IgG subtypes (but no IgM) failed to prevent a humoral immune response to transfused transgenic murine RBCs in double-KO mice lacking both FcγR and C3. In contrast, this antisera prevented an active humoral alloimmune response in wild-type and single-KO (FcγR KO or C3 KO) mice. KEL RBCs were observed to circulate for many days after transfusion in the double-KO mice, with passively administered anti-KEL remaining bound to all detectable KEL antigen sites. Given the robust immune response to KEL RBCs in FcγR × C3 double-KO mice in the presence of anti-KEL sera, these data make epitope masking an unlikely mechanism of action for anti-KEL immunoprophylaxis.
We posit instead that “antigenic clearance” or “antigen modulation” may be critically important to the mechanism of action of anti-KEL immunoprophylaxis. The KEL glycoprotein was essentially undetectable by flow cytometry within 24 hours after transfusion in all recipients studied (wild-type, FcγR KO, and C3 KO) in which anti-KEL immunoprophylaxis effectively prevented alloimmunization. Further, the fact that western blots of RBC membrane preps from these wild-type mice also had essentially undetectable amounts of the KEL glycoprotein implies that anti-KEL immunoprophylaxis led to removal of the entire KEL glycoprotein from the RBC. In contrast, the KEL glycoprotein remained detectable and unchanged in the peritransfusion period in double-KO mice lacking both FcγRs and C3, by both flow cytometry and western blot. RBCs expressing the highest levels of the KEL glycoprotein were presumably selectively cleared most rapidly in wild-type and single-KO mice, though a comparison of the clearance patterns to the antigen modulation patterns makes it unlikely that clearance alone is responsible for the decreased antigen detection. Antigen modulation, also known as “trogocytosis,” has been described to occur on white blood cells in response to monoclonal antibody therapy.24 Though less well described, antigen modulation has also been observed to occur on RBCs in mice and humans in the presence of auto- or alloantibodies.25 Modulation of the RhD antigen has even recently been observed, after RhIg was given to an RhD-positive patient with immune thrombocytopenic purpura.26 Taking past findings together with our current observations, antigen modulation (eg, removal of the entire KEL glycoprotein from the RBC membrane) may thus be an important consideration in the mechanism of action of anti-KEL in this murine model and also in immunoprophylaxis therapies against other antigenic targets.
At this time, we cannot exclude RBC clearance as being involved in the mechanism of action of anti-KEL immunoprophylaxis, as double-KO mice demonstrated less in vivo clearance of KEL RBCs than other strains evaluated and as the splenocytes from double-KO mice showed no in vitro phagocytosis of opsonized KEL RBCs. Many monoclonal anti–D antibodies historically were selected for additional study based on their ability to clear transfused RhD RBCs in human volunteers, though no reproducible correlation between RBC clearance patterns and the efficacy of immunoprophylaxis has emerged to date.11,27 Further, recent studies evaluating immunoprophylaxis in the HOD murine model also suggest that RBC clearance does not correlate with the efficacy of alloimmunization prevention,16 yet antigen modulation may complicate some clearance measurements.28 It thus remains to be determined whether KEL RBCs transfused in the presence of immunoprophylaxis need to be consumed by antigen-presenting cells in order for nonresponsiveness to be induced. Beyond phagocytosis by antigen-presenting cells, the roles of antigen presentation by different cell types and antigen trafficking to particular locations in the spleen and/or liver also remain to be determined. Future investigation of these issues, at the cellular and molecular levels, will be critical to better understand the phenomena described in this article.
Historically, studies have focused on the importance of FcγRs in consideration of the mechanism of action of immunoprophylaxis, as polyclonal anti-D (in RhIg) does not fix complement.29 However, emerging data refute the sole importance of FcγRs in immunoprophylaxis. For example, neither activating nor inhibitory receptors are critical for effective immunoprophylaxis against sheep RBCs.19 The applicability of data obtained in models involving sheep RBCs to those involving syngeneic or allogeneic RBCs is unclear, in light of the rapid baseline clearance of the xenogeneic RBCs that occurs even in the absence of anti–sheep RBC antibody.30 In a full mouse model, another group has recently described that IgG-mediated immune suppression to murine RBCs expressing the model HOD antigen occurs in the absence of activating or inhibitory FcγRs.18 Given these past sheep RBC and murine RBC studies, our finding that FcγRs are not required for effective immunoprophylaxis against transfused KEL RBCs was not unexpected. However, the fact that anti-KEL sera was not able to prevent alloimmunization in double-KO mice suggests that FcγRs are, in fact, important in some way, albeit in a way that appears to be fully compensated when C3 is present. It should also be mentioned that one prior study has documented alterations in immunomodulatory cytokines after RhIg treatment, with the impact of these cytokines on the immunoprophylaxis effect being unclear.31
The role of complement in antibody responses to non-RBC antigens has been of interest ever since studies in the 1970s showed impaired responses after depletion of complement by cobra venom factor.32 Antibody responses to suboptimal antigen doses in animals lacking C1q, C4, C2, C3, and CR1/2 have since been described to be impaired,33,34 thus making it historically difficult to study the importance of complement in antibody-mediated immune suppression. Mice lacking C1q, C3, or CR2 have poor antibody responses to transfused sheep RBCs, with a recent study demonstrating that IgG is able to suppress the antibody responses in these animals even further.19 Of note, mice lacking C3 in our studies had robust responses to transfused RBCs expressing the KEL antigen, potentially due to a lower threshold for B-cell activation in this model.35 A clear difference in humoral immune responses was thus able to be observed when C3 KO mice were transfused with KEL RBCs in the presence (no detectable humoral response) or absence (robust humoral response) of anti-KEL immunoprophylaxis. Of note, it has been hypothesized that deposition of complement products such as C3dg may be protective from future phagocytosis.36,37 Although studies in C3 KO mice demonstrated that this effect alone was not critical to the mechanism of action of anti-KEL immunoprophylaxis, the contributing role of such byproducts in the failure of anti-KEL immunoprophylaxis in double-KO mice remains to be determined.
Limitations to any study must be considered. These studies were completed in one murine model of alloimmunization using a single system, and factors such as antigen characteristics and antibody specificity differ by model. Moreover, the importance of the role of complement and FcγRs may vary by model, and it cannot be assumed that these results will be directly translatable to other animal models. Further, differences between murine and human IgG subtypes, FcγRs, and complement exist, and the potential pleotropic effects caused by a lack of FcγRs38 or C3 should be considered. Although we presume the entire KEL glycoprotein was fully modulated in wild-type animals in response to anti-KEL immunoprophylaxis, it must be noted that the antibodies used for both flow cytometry and western blot target the extracellular portion of the glycoprotein. These studies were focused on prevention of anti-KEL IgG responses, as IgG is the focus of testing by clinical transfusion medicine services and is the isotype most critical in hemolytic disease of the newborn. The contribution of complement receptor 1 or 2 on the mechanism of action of anti-KEL sera was not directly studied, nor was the contribution of the inhibitory receptor, FcγRIIb. These receptors are unlikely to be of critical importance in the KEL model, however, given results recently published in other systems.18,19
In conclusion, immunoprophylaxis with anti-KEL sera did not prevent alloimmunization to transfused KEL RBCs in double-KO recipients lacking both FcγR and C3, despite its efficacy in wild-type recipients and in single-KO recipients lacking either FcγR or C3. The observation that modulation of the KEL glycoprotein antigen did not occur in the peritransfusion period in double-KO mice provides new insight into the potential mechanism(s) of action of anti-RBC immunoprophylaxis. These findings may have broader implications for immunoprophylaxis development programs in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was funded by National Institutes of Health/National Heart, Lung, and Blood Institute grant R01 HL126076 (J.E.H.).
Authorship
Contribution: J.L., S.R.S., D.R.B., and J.E.H. planned and executed the experiments, and all authors made experimental suggestions; J.L. and J.E.H. wrote the initial draft of the manuscript, and all authors edited the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeanne E. Hendrickson, Yale University, Departments of Laboratory Medicine and Pediatrics, 330 Cedar St, Clinic Building 405, PO Box 208035, New Haven, CT 06520-8035; e-mail: jeanne.hendrickson@yale.edu.