Key Points
Dysfunctional BM EPCs were found in subjects with PGF postallotransplant.
BM EPCs from subjects with PGF were enhanced by atorvastatin through downregulation of the p38 MAPK pathway.
Abstract
Poor graft function (PGF) is a serious complication after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Murine studies suggest that endothelial progenitor cells (EPCs) are preferential supporting cells for hematopoietic stem cells in the bone marrow (BM) microenvironment. Our previous work found that a reduced number of BM EPCs was an independent risk factor for the occurrence of PGF after allo-HSCT. However, little is known about the functional role of BM EPCs and how to improve impaired BM EPCs in PGF. In the current study, we evaluated the function of BM EPCs in subjects with PGF postallotransplant. Moreover, we investigated whether atorvastatin could enhance the number and function of BM EPCs derived from subjects with PGF in vitro. Dysfunctional BM EPCs, which were characterized by impaired proliferation, migration, angiogenesis, and higher levels of reactive oxygen species and apoptosis, were revealed in subjects with PGF. Activation of p38 and its downstream transcription factor cyclic adenosine monophosphate–responsive element-binding protein were detected in BM EPCs from subjects with PGF. Furthermore, the number and function of BM EPCs derived from subjects with PGF were enhanced by atorvastatin treatment in vitro through downregulation of the p38 MAPK pathway. In summary, dysfunctional BM EPCs were observed in subjects with PGF. Atorvastatin treatment in vitro quantitatively and functionally improved BM EPCs derived from subjects with PGF through downregulation of the p38 MAPK pathway. These data indicate that atorvastatin represents a promising therapeutic approach for repairing impaired BM EPCs in subjects with PGF postallotransplant.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective treatment of malignant hematopoietic diseases. However, poor graft function (PGF) remains a serious complication after allo-HSCT, and the underlying mechanisms remain poorly understood.1-4 In murine studies, endothelial progenitor cells (EPCs) have been identified as a key cellular component supporting hematopoietic stem cells (HSCs) in the bone marrow (BM) microenvironment.5-8 In addition, our previous prospective nested case-control study suggested that the frequency of BM EPCs was markedly reduced in subjects with PGF. Multivariate analyses further identified reduced BM EPCs as an independent risk factor for the occurrence of PGF after allo-HSCT.2 However, the functional role of BM EPCs in the subjects with PGF has never been reported. Moreover, it remains largely unknown how to improve the dysfunction of BM EPCs in subjects with PGF.
BM-derived EPCs contribute to endothelial tissue repair and neovasculogenesis.8-10 By contrast, dysfunction of EPCs, which is characterized by changes in proliferation, migration, apoptosis, and angiogenesis, plays a pivotal role in the pathogenesis of multiple diseases, such as atherosclerosis, hypertension, diabetes, and heart failure.9,11 Moreover, reduced numbers of EPCs in the BM microenvironment have been associated with delayed hematopoietic recovery after transplantation in animal studies.12-14 Consequently, therapeutic strategies to improve the prognosis of subjects with PGF through enhancing the number and function of BM EPCs are urgently needed.
The therapeutic effects of atorvastatin, a synthetic 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, include lipid-lowering, antioxidant, anticoagulant, and anti-inflammatory activities, and so forth.15 Clinically, atorvastatin is widely used to treat dyslipidemia and associated vascular abnormalities. Furthermore, atorvastatin has been reported to improve the mobilization and function of circulating EPCs in a number of diseases, such as heart disease, diabetes, ischemic stroke, and others.16-18 Nevertheless, no previous studies have focused on the roles of atorvastatin on BM-derived EPCs in subjects with PGF following allo-HSCT.
MAPKs are a family of serine/threonine kinases that comprise 3 major subgroups, namely, extracellular signal-regulated kinase (ERK), p38 MAPK (p38), and JNK.19,20 The critical role of p38 MAPK and its downstream transcription factor cyclic adenosine monophosphate–responsive element-binding protein (CREB) in regulating the dysfunction of cultivated EPCs has been reported in coronary artery disease.19 Moreover, Dimmeler et al21 reported that 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) increase circulating EPCs via the phosphatidylinositol 3-kinase/Akt pathway. However, whether the p38 MAPK or Akt pathway is involved in regulating the dysfunction and repair of BM EPCs in subjects with PGF remains unknown.
In the current study, we evaluated the function of BM EPCs in subjects with PGF and investigated the effect of atorvastatin on the number and function of cultivated BM EPCs derived from subjects with PGF, as well as its underling molecular mechanisms.
Patients and methods
Subjects and controls
Three cohorts were enrolled: subjects with PGF, subjects with good graft function (GGF), and transplant donors as normal controls. Transplant recipients were identified from subjects (N = 578) who received an allotransplant from June 30 to December 31, 2015; from April 1 to July 8, 2016; and from August 19 to September 19, 2016 at Peking University Institute of Hematology. A total of 26 subjects who developed PGF were eligible. For each case, 1 matched transplant recipient with GGF was selected from the same cohort after matching for age, pretransplant disease state; and posttransplant interval (“risk-set sampling”).22 None of the clinical characteristics, such as the transplanted CD34+ cell dose, history of graft-versus-host disease (GVHD) or cytomegalovirus (CMV) infection, and anti-CMV therapy with ganciclovir, showed significant differences between subjects with PGF and GGF (Table 1).
BM samples from donors (N = 26) were normal controls. The normal cohort comprised 13 males and 13 females, aged 18 to 55 years (median, 42 years). The study was approved by the Ethics Committee of Peking University People’s Hospital, and written informed consent was obtained from all subjects, in compliance with the Declaration of Helsinki.
Definition of GGF/PGF
Transplant recipients had to have complete donor hematological chimerism with no residual or recurrent leukemia. GGF2-4,23 was characterized by an ANC >0.5 × 109/L for 3 consecutive days, PLT count >20 × 109/L for 7 consecutive days, and Hb level >70 g/L without transfusion support beyond day +28 post-HSCT. PGF2-4 was defined as a hypo- or aplastic BM with 2 or 3 of the following: (1) neutrophils ≤0.5 × 109/L; (2) PLTs ≤20 × 109/L; and/or (3) Hb concentration ≤70 g/L for at least 3 consecutive days after day +28 post-HSCT or in accordance with PLT and/or red blood cell transfusion and/or G-CSF support requirement. Patients with evidence of severe GVHD including grade III to IV aGVHD24 and severe chronic GVHD25 or hematological relapse after allo-HSCT were excluded.
Chimerism analyses were carried out by DNA fingerprinting for short tandem repeats in blood samples and/or by chromosome fluorescent in situ hybridization of BM samples. Complete donor chimerism was defined as the detection of no recipient hematopoietic or lymphoid cells (sensitivity >0.1% recipient signals).26
Transplantation protocols
Donor selection, HLA-typing, graft harvesting, conditioning therapy, and GVHD prophylaxis were performed as previously reported.26-29 The subjects were screened for CMV infection by serology. Real-time quantitative polymerase chain reaction was used to detect CMV reactivation twice a week in blood samples. CMV infection was treated with ganciclovir or foscarnet as described.30 After allo-HSCT, rhG-CSF (5 μg/kg per day) was administered to recipients of HLA-mismatched related transplants from day +6 until neutrophils were >0.5 × 109/L for 3 consecutive days. rhG-CSF was not administered to recipients of HLA-identical sibling transplants, except in cases where neutrophils were <0.5 × 109/L until day +21.
Isolation, cultivation, and characterization of primary BM EPCs
BM mononuclear cells (BMMNCs) were isolated by density gradient centrifugation using lymphocyte separation medium (GE Healthcare, Milwaukee, WI). BMMNCs (1 × 106 per well) were cultivated in fibronectin (Sigma, St. Louis, MO) precoated 24-well culture plates with EGM-2-MV-SingleQuots (Lonza, Walkersville, MD)31 and 10% fetal bovine serum (Gibco, MA)32 at 37°C in a humidified incubator with 5% CO2 for 7 days until testing.
After 7 days of cultivation, BM adherent cells were characterized as EPCs by detaching and staining with mouse anti-human CD34, CD133, and vascular endothelial growth factor receptor 2 (CD309) monoclonal antibodies (Becton Dickinson Biosciences, San Jose, CA), followed by detection using a BD LSRFortessa (Becton Dickinson).2,33,34 Aliquots of isotype-identical antibodies served as negative controls. Data were analyzed using BD LSRFortessa software (Becton Dickinson).
DiI-AcLDL uptake and FITC-UEA-I binding assay
After 7 days of cultivation, adherent cells were washed 3 times with phosphate-buffered saline and then incubated with 10 μg/mL Dil-Acetylated Low Density Lipoprotein (DiI-AcLDL; Life Technologies, Gaithersburg, MD) at 37°C. After 4 hours, the cells were washed and fixed in 4% prechilled paraformaldehyde for 10 minutes. After washes, the fixed cells were incubated for 1 hour with 10 μg/mL fluorescein isothiocyanate–labeled Ulex Europaeus Agglutinin-I (FITC-UEA-I, Sigma) at room temperature. Three power fields were randomly counted to evaluate the numbers of double positive staining EPCs per well using a fluorescence microscope (Olympus, Tokyo, Japan).
Cell counting
After treatment with 0.5 nM, 50 nM, 500 nM atorvastatin (Sigma); 10 μM p38 inhibitor (SB203580, Sigma); 400 nM p38 inhibitor (BIRB796, Selleck Chem, Houston, TX); and 1 mM N-acetyl-l-cysteine (NAC; Sigma) for 24 hours. The adherent BM EPCs at day 7 of cultivation were washed with phosphate-buffered saline and gently detached with trypsin containing 0.25% EDTA. The number of living cells per well was counted with trypan blue (Solarbio, Beijing, China) by independent blinded investigators.
Cell proliferation assay
Cell proliferation was measured using a cell counting kit-8 (Dojido, Kumamoto, Japan) assay according to the manufacturer's instructions. The final absorbance of each well was determined at 450 nM using a microplate reader (Bio-Rad, Foster City, CA).
Migration assay
Cell migration was assayed using a Transwell chamber (Corning, NY). The cells were trypsinized and seeded in the upper chambers at a density of 4 × 104 cells per well, while 500 μL medium was added to the lower chamber. The cells were cultured for 24 hours, and migrated cells were fixed with paraformaldehyde for 30 minutes. Then, cells on the bottom surface of the membrane were stained with crystal violet for 20 minutes. Cell images were obtained on a phase-contrast microscope (Olympus) and counted manually in 3 random fields/sample.
Tube formation assay
The plate and pipette tips used were prechilled, and each well was precoated with cold Matrigel (Corning, NY). A total of 4 × 104 EPCs at day 7 of cultivation were transferred to the Matrigel-coated plates for 48 hours at 37°C in 5% CO2. Tube formation was inspected on an inverted light microscope. The relative tube length per field of view was analyzed by Image Proplus and expressed as the mean ± standard error of the mean (SEM) by counting 3 random fields/sample.
Apoptosis analysis
To detect apoptosis, cells stained with the aforementioned EPC markers were incubated with the Annexin-V and 7-amino-actinomycin D Apoptosis Detection Kit (Becton Dickinson) according to the manufacturer’s instructions.
Measurement of intracellular reactive oxygen species (ROS) levels
Cells stained with the aforementioned EPCs markers were incubated with 10 μM 2’,7’-dichlorofluorescence diacetate (Byotimes, China) at 37°C for 15 minutes. The mean fluorescence intensity (MFI) of intracellular ROS was analyzed on a BD LSRFortessa (Becton Dickinson).4
Intracellular protein detection by flow cytometry
Intracellular proteins were detected by flow cytometry according to previously described techniques.4 Briefly, cells were incubated with EPC markers at 4°C for 30 minutes and then fixed, permeabilized, and incubated with phospho-p38, phospho-ERK, phospho-JNK, phospho-CREB, phospho-c-Jun (Cell Signaling Technology, Danvers, MA), and phospho-Akt antibodies (Becton Dickinson). Intracellular protein levels were analyzed using LSRFortessa software (Becton Dickinson) and expressed as the MFI (mean ± SEM).
Western blot analysis
Cells were harvested and lysed in radio-immunoprecipitation assay lysis buffer containing proteinase inhibitor and PhosSTOP (Roche, Indianapolis, IN). The proteins levels were quantified with a bicinchoninic acid assay for protein quantification kit (Thermo). Proteins were separated via 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (Millipore, Bedford, MA) membranes. The membranes were then blocked with 5% bovine serum albumin and incubated overnight with antibodies against phospho-p38 MAPK, total p38, phospho-ERK, total ERK, phospho-JNK, total JNK, phospho-Akt, total Akt, and glyceraldehyde-3-phosphate dehydrogenase (GADPH; Cell Signaling Technology) at dilutions specified by the manufacturer’s instructions. Horseradish peroxidase–conjugated secondary antibody was used in conjunction with an enhanced chemiluminescence detection system (Pierce Biotechnology Inc., Rockford, IL).
Coculture of BM CD34+ cells with BM EPCs
BM EPCs from subjects with PGF, GGF, or normal controls were cultivated for 7 days. Then, CD34+ cells were isolated from BMMNCs of healthy donors using a CD34 MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), placed in noncontact culture with StemSpan SFEM (Stem Cell Technologies, Vancouver, BC, Canada), and separated using 0.4-μm transwell inserts with adherent BM EPCs for another 7 days.35
Colony-forming unit (CFU) assays
CFUs were assayed using MethoCult H4434 Classic (Stem Cell Technologies). After 7 days of cultivation, nonadherent CD34+ cells were harvested, and 2 × 103 cells were plated in 24-well plates and cultured for 14 days. Colony-forming unit erythroid (CFU-E), burst-forming unit erythroid (BFU-E), colony-forming unit granulocyte-macrophage (CFU-GM), and colony-forming unit granulocyte, erythroid, macrophage, and megakaryocyte (CFU-GEMM) measurements were scored on an inverted light microscope. Cultures were assayed in duplicate, and results were expressed as the mean ± SEM.
Statistical analysis
Statistical analyses were performed using 1-way analysis of variance for comparisons among the groups. Subject variables were compared using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables. Analyses were performed using GraphPad Prism 6.0, and P values <.05 were considered statistically significant.
Results
Characterization of primary BM EPCs
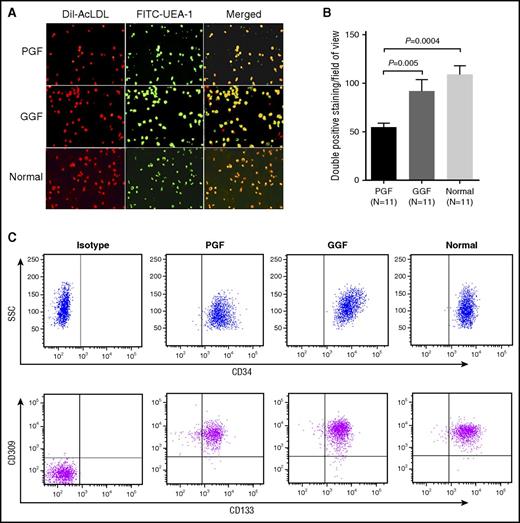
Spindle-shaped and elongated BM adherent cells at day 7 of cultivation were further functionally characterized as EPCs, which were capable of DiI-AcLDL uptake and FITC-UEA-I binding (Figure 1A). In addition, the typical EPC phenotype was confirmed by demonstrating similar expression of CD34, CD309, and CD133 in subjects with PGF and GGF and in normal controls by flow cytometry (Figure 1C).
Characterization of cultivated BM EPCs. (A) Representative images for cultivated BM EPCs at day 7 in culture among subjects with PGF and GGF and normal controls (Normal). Typical BM EPCs were characterized by double positive staining (merged in yellow) with both DiI-AcLDL (red) and FITC-UEA-I (green) (original magnification ×20). (B) Quantification of double positive staining cells (merged in yellow) with both DiI-AcLDL (red) and FITC-UEA-I (green) at day 7 in culture among the PGF, GGF, and normal control groups (original magnification ×10). Data are expressed as the mean ± SEM. (C) The typical EPCs phenotype of cultivated BM EPCs was confirmed by demonstrating the similar expression of CD34, CD309, and CD133 at day 7 in culture by flow cytometry among subjects with PGF, subjects with GGF, and normal controls. Aliquots of isotype-identical antibodies served as negative controls.
Characterization of cultivated BM EPCs. (A) Representative images for cultivated BM EPCs at day 7 in culture among subjects with PGF and GGF and normal controls (Normal). Typical BM EPCs were characterized by double positive staining (merged in yellow) with both DiI-AcLDL (red) and FITC-UEA-I (green) (original magnification ×20). (B) Quantification of double positive staining cells (merged in yellow) with both DiI-AcLDL (red) and FITC-UEA-I (green) at day 7 in culture among the PGF, GGF, and normal control groups (original magnification ×10). Data are expressed as the mean ± SEM. (C) The typical EPCs phenotype of cultivated BM EPCs was confirmed by demonstrating the similar expression of CD34, CD309, and CD133 at day 7 in culture by flow cytometry among subjects with PGF, subjects with GGF, and normal controls. Aliquots of isotype-identical antibodies served as negative controls.
Impaired function of BM EPCs in subjects with PGF
To evaluate primary BM EPCs function, double positive staining of DiI-AcLDL and FITC-UEA-I, angiogenic potential (including migration and tube formation), apoptosis, and intracellular ROS levels were analyzed.
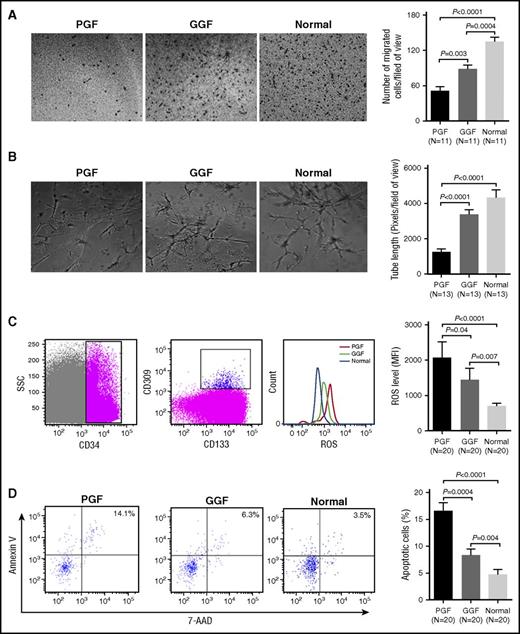
At day 7 of cultivation, BM EPCs from subjects with PGF showed significantly less double positive staining (Figure 1B; 54.2 ± 5.1 vs 91.7 ± 12.3; P = .005), as well as reduced migration (Figure 2A; 51.3 ± 7.3 vs 88.8 ± 6.0; P = .003) and tube formation (Figure 2B; 1265 ± 175.6 vs 3395 ± 293.2; P < .0001) compared with those from subjects with GGF.
Impaired function of BM EPCs in PGF subjects compared with GGF subjects and normal controls. (A) Representative images (left) and quantification (right) of the transwell migration assays of cultivated BM EPCs at day 7 in culture (original magnification ×10). The numbers of migrated BM EPCs per field of view were compared among subjects with PGF, subjects with GGF, and normal controls. Three power fields were randomly counted and averaged per sample. (B) Representative images (left) and quantification (right) of tube formation (pixels of tubes per field of view) by cultivated BM EPCs at day 7 in culture (original magnification ×10). Three power fields were randomly counted and averaged per sample. (C) To detect the levels of intracellular ROS or apoptosis in precultivated BMMNCs, the BM EPCs demonstrating the typical expression of CD34, CD309, and CD133 were firstly gated by flow cytometry (left). Representative images (middle) and quantification (right) of intracellular ROS levels in the gated precultivated BM EPCs. (D) Representative images (left) and quantification (right) of the apoptosis in the gated precultivated BM EPCs. Data are expressed as the mean and SEM.
Impaired function of BM EPCs in PGF subjects compared with GGF subjects and normal controls. (A) Representative images (left) and quantification (right) of the transwell migration assays of cultivated BM EPCs at day 7 in culture (original magnification ×10). The numbers of migrated BM EPCs per field of view were compared among subjects with PGF, subjects with GGF, and normal controls. Three power fields were randomly counted and averaged per sample. (B) Representative images (left) and quantification (right) of tube formation (pixels of tubes per field of view) by cultivated BM EPCs at day 7 in culture (original magnification ×10). Three power fields were randomly counted and averaged per sample. (C) To detect the levels of intracellular ROS or apoptosis in precultivated BMMNCs, the BM EPCs demonstrating the typical expression of CD34, CD309, and CD133 were firstly gated by flow cytometry (left). Representative images (middle) and quantification (right) of intracellular ROS levels in the gated precultivated BM EPCs. (D) Representative images (left) and quantification (right) of the apoptosis in the gated precultivated BM EPCs. Data are expressed as the mean and SEM.
Phospho-p38 MAPK and phospho-CREB were elevated in BM EPCs from subjects with PGF
To explore the involvement of the MAPK or Akt pathways in BM EPCs dysfunction in subjects with PGF, we examined the basal levels of phospho-p38, phospho-ERK, phospho-JNK, and phospho-Akt in precultivated BM EPCs by flow cytometry, as well as via western blot in cultivated BM EPCs at day 7, derived from subjects with PGF, subjects with GGF, and normal controls.
Using flow cytometry, we detected remarkably higher levels of phospho-p38 in BM EPCs from subjects with PGF than in those from subjects with GGF (Figure 3A; 2857 ± 320.5 vs 1715 ± 77.0; P = .003). In contrast, intracellular phospho-ERK (Figure 3B; 2347 ± 466.7 vs 2035 ± 465.0; P = .64), phospho-JNK (Figure 3C; 1380 ± 224.2 vs 1421 ± 213.3; P = .88), and phospho-Akt levels (Figure 3D; 181.4 ± 12.4 vs 166.5 ± 16.5; P = .13) were not significantly different between subjects with PGF and GGF. Likewise, the basal expression patterns of these intracellular proteins detected by flow cytometry were further validated in cultivated BM EPCs at day 7 by western blot (Figure 3G).
Elevated levels of intracellular phospho-p38 and its downstream transcription factor phospho-CREB in BM EPCs from PGF subjects compared with GGF subjects and normal controls. Flow cytometry revealed increased phospho-p38 (A) and phospho-CREB (E), whereas no significant differences in phospho-ERK (B), phospho-JNK (C), phospho-Akt (D), and phospho-c-Jun (F) expression in precultivated BM EPCs among subjects with PGF, subjects with GGF, and normal controls. Data are expressed as the MFI (mean ± SEM). (G) Representative western blots of phospho-p38, total p38, phospho-ERK, total ERK, phospho-JNK, total JNK, phospho-Akt, total Akt, and GADPH in cultivated BM EPCs at day 7 in culture among subjects with PGF, subjects with GGF, and normal controls.
Elevated levels of intracellular phospho-p38 and its downstream transcription factor phospho-CREB in BM EPCs from PGF subjects compared with GGF subjects and normal controls. Flow cytometry revealed increased phospho-p38 (A) and phospho-CREB (E), whereas no significant differences in phospho-ERK (B), phospho-JNK (C), phospho-Akt (D), and phospho-c-Jun (F) expression in precultivated BM EPCs among subjects with PGF, subjects with GGF, and normal controls. Data are expressed as the MFI (mean ± SEM). (G) Representative western blots of phospho-p38, total p38, phospho-ERK, total ERK, phospho-JNK, total JNK, phospho-Akt, total Akt, and GADPH in cultivated BM EPCs at day 7 in culture among subjects with PGF, subjects with GGF, and normal controls.
To further explore the molecular basis of BM EPCs dysfunction in subjects with PGF, the downstream targets of phospho-p38 (eg, CREB, c-Jun) were analyzed by flow cytometry. Markedly higher phospho-CREB expression (Figure 3E; 902.8 ± 113.6 vs 374.6 ± 54.1; P = .0006) was observed in subjects with PGF than in subjects with GGF. However, no significant differences were found in intracellular phospho-c-Jun (Figure 3F; 219.0 ± 38.0 vs 220.5 ± 46.2; P = .65) expression between subjects with PGF and GGF.
Atorvastatin improved the number and function of BM EPCs in subjects with PGF
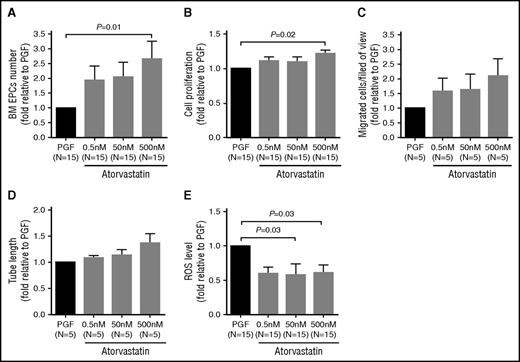
We first investigated the dose-response effect of atorvastatin (0.5, 50, and 500 nM) on the number and function of cultivated BM EPCs at day 7 in subjects with PGF. BM EPCs from subjects with PGF were quantitatively and functionally improved by atorvastatin, especially at 500 nM (Figure 4).
Dose-response effects of atorvastatin treatment on the number and function of cultivated BM EPCs from PGF subjects. BM EPCs from PGF subjects were incubated with atorvastatin (0.5 nM, 50 nM, 500 nM). The dose-response effects of atorvastatin treatment on cell number (per well) (A), cell proliferation (B), migration (C), tube formation (D), and ROS levels (E) of cultivated EPCs from PGF subjects were evaluated at day 7 in culture. Data are expressed as fold-change relative to PGF. All of the P values < .05 were considered statistically significant and provided in the panels. The changes were not statistically significant in panels C and D.
Dose-response effects of atorvastatin treatment on the number and function of cultivated BM EPCs from PGF subjects. BM EPCs from PGF subjects were incubated with atorvastatin (0.5 nM, 50 nM, 500 nM). The dose-response effects of atorvastatin treatment on cell number (per well) (A), cell proliferation (B), migration (C), tube formation (D), and ROS levels (E) of cultivated EPCs from PGF subjects were evaluated at day 7 in culture. Data are expressed as fold-change relative to PGF. All of the P values < .05 were considered statistically significant and provided in the panels. The changes were not statistically significant in panels C and D.
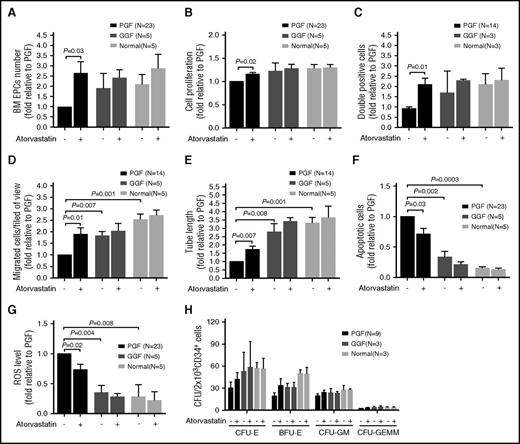
Atorvastatin (500 nM) significantly increased the number (Figure 5A; 2.64 ± 0.57-fold; P = .03) and proliferation rate (Figure 5B; 1.16 ± 0.03-fold; P = .02) of BM EPCs in subjects with PGF compared with untreated cells. Moreover, atorvastatin (500 nM) improved the function of BM EPCs in subjects with PGF, including double positive staining cells (Figure 5C; 2.07 ± 0.36-fold; P = .01), migration capacity (Figure 5D; 1.91 ± 0.27-fold; P = .01), and tube formation capability (Figure 5E; 1.74 ± 0.17-fold; P = .007). Reduced levels of apoptosis (Figure 5F; 0.71 ± 0.09-fold; P = .03) and intracellular ROS (Figure 5G; 0.74 ± 0.10-fold; P = .02) were also observed in the atorvastatin (500 nM) treatment group.
In vitro treatment with atorvastatin, p38 inhibitors, and ROS inhibitor demonstrated similar effects on the number and function of cultivated BM EPCs from PGF subjects. The cultivated BM EPCs from PGF subjects were incubated with atorvastatin (500 nM), NAC (1 mM), SB203580 (10 μM), or BIRB796 (400 nM). The effects of the different treatments on cell number (per well) (A), cell proliferation (B), double positive staining cells (per well) (C), migration (D), tube formation (E), apoptosis (F), and ROS levels (G) of cultivated BM EPCs from PGF subjects were compared at day 7 in culture. Data are expressed as fold-change relative to PGF.
In vitro treatment with atorvastatin, p38 inhibitors, and ROS inhibitor demonstrated similar effects on the number and function of cultivated BM EPCs from PGF subjects. The cultivated BM EPCs from PGF subjects were incubated with atorvastatin (500 nM), NAC (1 mM), SB203580 (10 μM), or BIRB796 (400 nM). The effects of the different treatments on cell number (per well) (A), cell proliferation (B), double positive staining cells (per well) (C), migration (D), tube formation (E), apoptosis (F), and ROS levels (G) of cultivated BM EPCs from PGF subjects were compared at day 7 in culture. Data are expressed as fold-change relative to PGF.
Atorvastatin, ROS inhibitor, and p38 inhibitors similarly affected the number and function of BM EPCs in subjects with PGF
Because elevated intracellular ROS and p38 MAPK levels were demonstrated in the BM EPCs from subjects with PGF, we next evaluated the effects of inhibiting ROS or p38 MAPK using the antioxidant NAC or p38 inhibitors (SB203580 and BIRB796), respectively, on the number and function of cultivated BM EPCs from subjects with PGF.
Treatment in vitro with SB203580 (1.93 ± 0.30-fold; P = .02) significantly increased the migration capability (Figure 5D) of BM EPCs in subjects with PGF. Both NAC (1.76 ± 0.23-fold; P = .03) and SB203580 (1.80 ± 0.29-fold; P = .02) improved tube formation capability (Figure 5E). Moreover, NAC (0.48 ± 0.04-fold; P = .003) and SB203580 (0.46 ± 0.09-fold; P = .004) attenuated apoptosis (Figure 5F) of BM EPCs from subjects with PGF. NAC (0.62 ± 0.22-fold; P = .01) and SB203580 (0.66 ± 0.18-fold; P = .01) reduced ROS level (Figure 5G). Another p38 inhibitor (BIRB796) produced similar effects to SB203580 on the number and function of BM EPCs in subjects with PGF (Figure 5). These findings indicate that inhibiting ROS or p38 activation enhances the number and function of BM EPCs in subjects with PGF, similar to atorvastatin treatment.
Atorvastatin produced superior effects on the number and function of BM EPCs from subjects with PGF compared with those from subjects with GGF or from normal controls
Because BM EPCs from subjects with PGF were found to be quantitatively and functionally impaired, we hypothesized that atorvastatin may have different effects on the BM EPCs of different origin. To test this hypothesis, we compared the effects of atorvastatin on the BM EPCs derived from subjects with PGF with those from subjects with GGF or from normal controls.
Notably, in vitro treatment with atorvastatin significantly increased the number as well as the proliferative capacity, double positive staining, migration, and tube formation of BM EPCs from subjects with PGF compared with those from subjects with GGF or from normal controls (Figure 6A-E). In addition, atorvastatin treatment led to a greater reduction in apoptosis and ROS in BM EPCs from subjects with PGF compared with those from subjects with GGF or from normal controls (Figure 6F-G). These data suggest that BM EPCs from subjects with PGF are more sensitive to atorvastatin than those from subjects with GGF or from normal controls.
Atorvastatin displayed superior effects on the number and function of BM EPCs from PGF subjects compared with GGF subjects or normal controls. The cultivated BM EPCs from PGF or GGF subjects or normal controls were incubated with atorvastatin (500 nM). The effects of atorvastatin treatment on the cell number (A), cell proliferation (B), double positive staining cells (per well) (C), migration (D), tube formation (E), apoptosis (F), and ROS levels (G) of cultivated BM EPCs from PGF or GGF subjects or normal controls were compared at day 7 in culture. (H) CFU plating efficiency in CD34+ BM cells was analyzed after coculturing with the differently treated BM EPCs from PGF or GGF subjects or normal controls. The CFU outgrowth was improved with atorvastatin, but the results were not statistically significant. Data are expressed as the fold-change relative to PGF.
Atorvastatin displayed superior effects on the number and function of BM EPCs from PGF subjects compared with GGF subjects or normal controls. The cultivated BM EPCs from PGF or GGF subjects or normal controls were incubated with atorvastatin (500 nM). The effects of atorvastatin treatment on the cell number (A), cell proliferation (B), double positive staining cells (per well) (C), migration (D), tube formation (E), apoptosis (F), and ROS levels (G) of cultivated BM EPCs from PGF or GGF subjects or normal controls were compared at day 7 in culture. (H) CFU plating efficiency in CD34+ BM cells was analyzed after coculturing with the differently treated BM EPCs from PGF or GGF subjects or normal controls. The CFU outgrowth was improved with atorvastatin, but the results were not statistically significant. Data are expressed as the fold-change relative to PGF.
Atorvastatin improved CFU plating efficiency in CD34+ BM cells cocultured with BM EPCs from subjects with PGF
To explore whether atorvastatin would also affect the ability of impaired BM EPCs to support hematopoietic progenitors in vitro, BM CD34+ cells from healthy donors were cocultured with cultivated BM EPCs from 3 groups of subjects. As shown in Figure 6H, the BM EPCs from subjects with PGF had a deficit in CFU outgrowth at baseline level compared with subjects with GGF, as determined by CFU-E (30.2 ± 8.3 vs 52.7 ± 26.3; P = .37), BFU-E (19.1 ± 4.5 vs 30.7 ± 5.7; P = .21), CFU-GM (19.3 ± 2.9 vs 23.0 ± 6.4; P = .52), and CFU-GEMM (2.4 ± 0.3 vs 3.3 ± 1.9; P = .85). Moreover, atorvastatin (500 nM) exposure improved the ability of BM EPCs from subjects with PGF to support CD34+ hematopoietic progenitors from healthy donors, but the results were not statistically significant, as determined by CFU-E (41.9 ± 9.3 vs 30.2 ± 8.3; P = .26), BFU-E (33.3 ± 9.7 vs 19.1 ± 4.5; P = .20), CFU-GM (24.2 ± 2.9 vs 19.3 ± 2.9; P = .35), and CFU-GEMM (3.0 ± 0.7 vs 2.4 ± 0.3; P = .86).
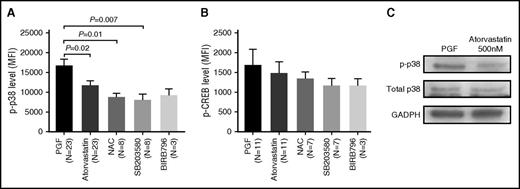
Atorvastatin reduced phospho-p38 MAPK activity in BM EPCs from subjects with PGF
To understand how atorvastatin affects the impaired BM EPCs from subjects with PGF, we analyzed the expression of intracellular proteins in BM EPCs treated with atorvastatin (500 nM) in vitro.
The higher phospho-p38 expression in BM EPCs from subjects with PGF decreased significantly following atorvastatin treatment (Figure 7A; 16 680 ± 1708 vs 11 610 ± 1243; P = .02), which was confirmed by western blot (Figure 7C). In addition, phospho-CREB, one of the downstream transcription factors of p38 MAPK, was also attenuated by atorvastatin in subjects with PGF (Figure 7B).
Atorvastatin reduced phospho-p38 MAPK activity in cultivated BM EPCs from PGF subjects. The cultivated BM EPCs from PGF subjects were incubated with atorvastatin (500 nM). Phospho-p38 (A) and phospho-CREB (B) expression was analyzed by flow cytometry in the cultivated BM EPCs at day 7 in culture. Data are expressed as the MFI (mean ± SEM). (C) Representative western blots of phospho-p38, total p38, and GADPH from cultivated BM EPCs at day 7 in culture among subjects with PGF, subjects with GGF, and normal controls.
Atorvastatin reduced phospho-p38 MAPK activity in cultivated BM EPCs from PGF subjects. The cultivated BM EPCs from PGF subjects were incubated with atorvastatin (500 nM). Phospho-p38 (A) and phospho-CREB (B) expression was analyzed by flow cytometry in the cultivated BM EPCs at day 7 in culture. Data are expressed as the MFI (mean ± SEM). (C) Representative western blots of phospho-p38, total p38, and GADPH from cultivated BM EPCs at day 7 in culture among subjects with PGF, subjects with GGF, and normal controls.
Discussion
The current study is the first to demonstrate the impaired function of BM EPCs, characterized by decreased capacities of proliferation, migration, and angiogenesis, as well as higher levels of ROS and apoptosis, in subjects with PGF following allo-HSCT. Moreover, BM EPCs derived from subjects with PGF exhibited quantitative and functional improvement following in vitro treatment with atorvastatin, and these effects were mediated through the downregulation of the p38 MAPK pathway.
Emerging evidence from murine studies suggests that BM EPCs are among the preferential supporting cells for HSCs in BM microenvironment.5-8 Conditional deletion of vascular endothelial growth factor receptor 2 in irradiated mice was shown to block the regeneration of EPCs and prevent hematopoietic reconstitution,6 whereas the systemic administration of endothelial cells was shown to accelerate hematopoietic recovery and survival in lethally irradiated mice.12 Based on our previous work2 and the current study, BM EPCs were quantitatively and functionally impaired in subjects with PGF following allo-HSCT. Considering the predominant role of the BM EPCs in supporting hematopoiesis,5-8 we speculate that impaired BM EPCs may hamper the hematopoietic reconstitution of successfully engrafted donor HSCs, ultimately leading to the occurrence of PGF postallotransplant.
Direct infusion of circulating EPCs can improve endothelial repair and significantly increase BM HSCs.13 Such infusion also accelerates hematopoietic and immune reconstitution after allo-HSCT in mice.36 However, the critical limitation for the therapeutic application of EPCs infusion in humans is their low number in circulation.37 Therefore, the mobilization of endogenous BM-derived EPCs via exogenous agents such as statins is considered as a potential therapeutic strategy to regulate vascular homeostasis and hematopoiesis.
Recent studies have demonstrated that statin therapy can enhance the number and function of EPCs in vitro, in animals, and in human subjects with cardiovascular diseases by downregulating p38 MAPK19,20 or upregulating Akt signaling.21,38,39 We provide experimental evidence that the dysfunction of BM EPCs from subjects with PGF after allo-HSCT is related to the activation of the p38 MAPK pathway and its downstream transcription factor CREB. By contrast, no significant differences were detected in phospho-JNK, ERK, or Akt levels between subjects with PGF and GGF. Furthermore, the impaired BM EPCs in subjects with PGF were quantitatively and functionally improved by atorvastatin in vitro, potentially through the downregulation of the p38 MAPK pathway. Therefore, atorvastatin, as a regulator of p38 MAPK, appears to be an attractive therapeutic target to optimize the BM microenvironment in subjects with PGF. Indeed, our preliminary data revealed that atorvastatin can improve the ability of impaired BM EPCs to support hematopoietic progenitors, although without statistical significance, as determined by CFU plating efficiency in vitro.
During early embryogenesis, EPCs and HSCs likely arise from a common precursor cell.37,40 Therefore, HSCs probably also provide survival and proliferation signals for EPCs. However, whether BM EPCs dysfunction in subjects with PGF is responsible for PGF, or vice versa, requires further clarification.
In summary, BM EPCs dysfunction was demonstrated in subjects with PGF, and these impairments could be both quantitatively and functionally improved by atorvastatin treatment in vitro through the downregulation of the p38 MAPK pathway. Therefore, our data indicate that it would be of value to investigate whether atorvastatin treatment can improve hematopoietic reconstitution through bolstering BM EPCs in subjects with PGF postallotransplant in phase 1/2 clinical trials in the future.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the core facilities at Peking University Institute of Hematology for sample collection.
This work was supported by the National Natural Science Foundation of China (grants 81370638, 81570127, 81530046, and 81230013), the Beijing Municipal Science and Technology Program (grants Z151100004015164, Z151100001615020, and Z141100000214011), Milstein Medical Asian American Partnership Foundation, the Science and Technology Project of Guangdong Province of China (grant 2016B030230003), and the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant 81621001). American Journal Experts (http://www.journalexperts.com) provided editorial assistance to the authors during the preparation of the manuscript.
Authorship
Contribution: X.-J.H. and Y.K. designed the study and supervised the manuscript preparation; M.-M.S. and Y.K. performed the research, analyzed the data, and wrote the manuscript; all other authors participated in the collection of patients’ data and agreed to submit the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao-Jun Huang, Peking University People’s Hospital, Peking University Institute of Hematology, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Collaborative Innovation Center of Hematology, Peking University, No. 11 Xizhimen South St, Beijing 100044, China; e-mail: xjhrm@medmail.com.cn.
References
Author notes
M.-M.S. and Y.K. contributed equally to this study.