Key Points
The widely used diabetes drug metformin improves hematopoiesis and delays tumor formation in a preclinical murine model of FA.
Metformin reduces DNA damage in human FA patient–derived cells.
Abstract
Fanconi anemia (FA) is an inherited bone marrow failure disorder associated with a high incidence of leukemia and solid tumors. Bone marrow transplantation is currently the only curative therapy for the hematopoietic complications of this disorder. However, long-term morbidity and mortality remain very high, and new therapeutics are badly needed. Here we show that the widely used diabetes drug metformin improves hematopoiesis and delays tumor formation in Fancd2−/− mice. Metformin is the first compound reported to improve both of these FA phenotypes. Importantly, the beneficial effects are specific to FA mice and are not seen in the wild-type controls. In this preclinical model of FA, metformin outperformed the current standard of care, oxymetholone, by improving peripheral blood counts in Fancd2−/− mice significantly faster. Metformin increased the size of the hematopoietic stem cell compartment and enhanced quiescence in hematopoietic stem and progenitor cells. In tumor-prone Fancd2−/−Trp53+/− mice, metformin delayed the onset of tumors and significantly extended the tumor-free survival time. In addition, we found that metformin and the structurally related compound aminoguanidine reduced DNA damage and ameliorated spontaneous chromosome breakage and radials in human FA patient–derived cells. Our results also indicate that aldehyde detoxification might be one of the mechanisms by which metformin reduces DNA damage in FA cells.
Introduction
Fanconi anemia (FA) is an inherited bone marrow failure disorder associated with a high incidence of leukemia and solid tumors.1 The disorder is caused by a disrupted FA-BRCA pathway and is genetically heterogeneous, with at least 21 complementation groups and genes (FANCA, FANCB, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCJ/BRIP1/BACH1, FANCL, FANCM, FANCN/PALB2, FANCO/RAD51C, FANCP/SLX4, FANCQ/XPF/ERCC4, FANCR/RAD51, FANCS/BRCA1, FANCT/UBE2T, FANCU/XRCC2, and FANCV/MAD2L2/REV7) identified thus far. The main role of this gene network is to repair DNA lesions such as interstrand cross-links, which impede replication and transcription.2,3
The primary cause of early morbidity and mortality for FA patients is bone marrow failure.4 Hematopoietic stem cells (HSCs) in FA patients are reduced in number, function poorly compared with healthy HSCs, and also suffer from progressive elimination because of the accumulation of unrepaired DNA damage.5,6 Although most strains of FA mutant mice are poor models of the human disease, Fancd2−/− mice recapitulate the key human disease phenotypes well, including HSC defects and progressive bone marrow failure.7,8 Fancd2−/− mice display thrombocytopenia by 3 to 6 months of age and eventually progress to peripheral pancytopenia by 18 months.9 Fancd2−/− HSCs are less quiescent and show a severely reduced capacity to repopulate the hematopoietic system in vivo.8
The FA pathway plays a fundamental role in protecting cells against DNA damage–inducing aldehydes.10 Disruption of key aldehyde detoxifying enzymes such as the aldehyde dehydrogenases Aldh2 or Adh5 in Fanconi mice induces phenotypes resembling clinical FA and leads to spontaneous bone marrow failure.11,12 Of note, human FA patients carrying a dominant-negative allele of ALDH2 demonstrate accelerated progression of bone marrow failure.13 These observations suggest that attenuating aldehyde toxicity may provide a novel therapeutic approach to FA. Metformin (N,N-dimethylbiguanide) is a widely used drug to treat diabetes with proven safety after decades of clinical use.14 As a guanidine derivative, metformin has the potential to scavenge DNA damage–inducing aldehydes through the Mannich reaction.15 Metformin also induces the activation of adenosine 5′-monophosphate–activated protein kinase (AMPK) and is thought to have its antidiabetic effect via this mechanism.16,17 We have reported that the plant polyphenol resveratrol helps to restore the quiescence of Fancd2−/− HSCs and improves the function of hematopoietic stem and progenitor cells (HSPCs) in these mice.8,18 Resveratrol has several bioactivities, including acting as an antioxidant, activating Sirt deacetylases (sirtuins), and activating AMPK.19 However, we have recently demonstrated that a potent sirtuin activator, SRT3025, does not mimic the effects of resveratrol in FA mice.20 Given that AMPK plays an important role in HSCs,21 AMPK activation may be the primary mechanism by which resveratrol improves hematopoiesis. The ability of metformin to activate AMPK and act as a potential aldehyde scavenger makes metformin a potential candidate for the treatment of FA. In the current study, we tested the effects of chronic metformin therapy on hematopoiesis and cancer incidence in Fancd2−/− mice.
Materials and methods
Mice
Fancd2 mutant mice were maintained on the 129S4 background.22 The metformin diet was made by milling metformin with standard rodent diet (Purina Chow 5001) at 3.75 g/kg diet (Bio-Serv, Flemington, NJ) and was administered to mice upon weaning (3-4 weeks of age). The treatment lasted 6 months unless specified otherwise. All animals were treated in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Polyinosinic:polycytidylic acid [poly(I:C)] was purchased from GE Healthcare (Piscataway, NJ) and given to the mice at 8 mg/kg body weight via intraperitoneal injection. Control mice were injected with saline.
CBC
Blood samples were collected in EDTA-coated capillary tubes and complete blood counts (CBCs) were measured on a Hemavet 950FS Multi-species Hematology System (Drew Scientific, Inc, Dallas, TX).
Flow cytometry
Bone marrow cells were isolated from the femora and tibiae of mice and stained as described previously.8 The c-Kit+Sca-1+Lin− (KSL) antibody cocktail was composed of anti-mouse c-Kit, Sca-1, and lineage markers (CD3e, CD4, CD5, CD8a, B220, Ter119, NK1.1, Mac1, and Gr1). For analysis of CD34−KSL cells, nucleated bone marrow cells were stained with anti-mouse CD34 along with the KSL antibody cocktail. All the antibodies were from eBioscience (San Diego, CA). Flow cytometry analysis was performed on a Cytopeia Influx cell sorter.
CFU-S assay
Colony-forming unit–spleen (CFU-S) assay was performed as described previously8 (see also supplemental Methods, available on the Blood Web site).
Cells and reagents
PD259i fibroblast cells, provided by the Oregon Health & Science University FA Cell Repository (http://www.ohsu.edu/research/fanconi-anemia/celllines.cfm), were originally derived from a human FA-A patient. EUFA316 lymphoblastoid cells were originally derived from a human FA-G patient.23 EUFA316+FANCG cells were modified EUFA316 cells that stably express the wild-type FANCG.24
Metformin and aminoguanidine were purchased from MP Biomedicals (Santa Ana, CA) and Tokyo Chemical Industry (Tokyo, Japan), respectively. The Adh5 inhibitor C3 compound was obtained from ChemDiv (San Diego, CA).
Radial and chromosomal breakage assay
PD259i cells were treated with either metformin, aminoguanidine, or placebo. In the case when the C3 compound was used for the assay, C3 was added 1 hour after the addition of metformin. Forty-eight hours later, metaphase spreads were made and scored for radial contents and chromosomal breakage on a Zeiss Axioskop photoscope.
Statistical analysis
Unless specified otherwise, the 2-tailed, unpaired Student t test was used for statistical analysis. A value of P < .05 was considered significant.
Results
Dietary metformin administration enhances hematopoiesis
To determine whether metformin can influence hematopoiesis, cohorts of Fancd2−/− and wild-type mice were given either metformin-supplemented rodent chow or placebo for 6 months beginning at 1 month of age. The food intake was measured, and the effective dose via ingestion was calculated to be 300 mg/kg per day. On the basis of the body surface area conversion,25 this dose was equivalent to only ∼65% of the maximum dose used routinely in humans (∼1300 mg/m2). After 6 months, CBCs were examined. Fancd2−/− mice on the placebo diet showed mild pancytopenia in multiple lineages, including lower platelet counts, lower white and red blood cell counts, and lower hemoglobin levels than their wild-type gender-matched littermate placebo controls (Table 1). A CBC analysis revealed that chronic metformin treatment significantly increased platelet counts (P < .05; Figure 1A) in Fancd2−/− mice, but not in wild-type controls. In contrast, white blood cell counts (P < .05; Figure 1A) increased in both metformin-treated Fancd2−/− and wild-type mice. Although metformin-treated Fancd2−/− mice showed only a mild and nonsignificant increase in red blood cell number (P = .08; Figure 1A), the hemoglobin levels in metformin-treated Fancd2−/− mice were significantly higher than those in placebo-treated Fancd2−/− mice (P < .005; Figure 1A), nearly comparable to those observed in the placebo-treated wild-type controls. These multilineage improvements in hematologic parameters took place significantly faster with metformin treatment as opposed to oxymetholone treatment, the current standard androgen treatment of FA patients, on the same Fancd2−/− murine model tested in our previous studies.9,20
Metformin administration enhances hematopoiesis. (A) CBCs after 6 months of treatment with metformin. D2, Fancd2; HGB, hemoglobin; NS, not significant. The data are pooled results from 17 to 19 individual mice in each group. (B) Representative flow cytometry profiles for placebo and metformin-treated Fancd2−/− mice. The percentages on the profiles indicate the mean value for each group. PI, propidium iodide. (C) Quantification of CD34−KSL frequency in bone marrow. The data represent the percentage of CD34−KSL cells in all nucleated bone marrow cells from 15 mice in each group.
Metformin administration enhances hematopoiesis. (A) CBCs after 6 months of treatment with metformin. D2, Fancd2; HGB, hemoglobin; NS, not significant. The data are pooled results from 17 to 19 individual mice in each group. (B) Representative flow cytometry profiles for placebo and metformin-treated Fancd2−/− mice. The percentages on the profiles indicate the mean value for each group. PI, propidium iodide. (C) Quantification of CD34−KSL frequency in bone marrow. The data represent the percentage of CD34−KSL cells in all nucleated bone marrow cells from 15 mice in each group.
We next focused on characterizing the bone marrow of mice in this cohort. The marrow cellularity in either Fancd2−/− or wild-type mice was not different between metformin-treated animals and placebo-treated controls (data not shown). Interestingly, flow cytometry analysis demonstrated that the size of the CD34−KSL cell compartment, an immunophenotypically defined HSC population in Fancd2−/− mice (Figure 1B), was significantly increased by 48% after 6 months of chronic metformin administration (Figure 1C). The size of the CD34−KSL cell compartment in wild-type mice, in contrast, was unchanged (Figure 1C), indicating that the effect of metformin on HSC population size was specific to Fancd2−/− mice.
Because our previous study showed that the AMPK activator resveratrol could help maintain stem cell quiescence in Fancd2−/− mice,8 we measured the impact of metformin on the cell cycle status of HSPCs. The cell cycle profiles of KSL cells from metformin-treated mice were examined using Hoechst 33342 staining (for DNA content) and intracellular Ki67 staining (to discriminate cycling G1 cells from noncycling G0 cells).26 As shown in Figure 2A-B, the average frequency of quiescent G0 KSL cells in metformin-treated Fancd2−/− mice was 27.4%, which was substantially higher (P < .05) than the average G0 fraction of 20.3% observed in placebo-treated gender-matched Fancd2−/− mice. Correspondingly, the average S-G2-M proportion of KSL cells in metformin-treated Fancd2−/− mice was 21.2%, which was significantly lower (P < .05) than the average S-G2-M percentage of 25.7% observed in placebo-treated controls. These results indicate that metformin treatment increased the quiescence of HSPCs in Fancd2−/− mice. In contrast, the cell cycle status of KSL cells in wild-type mice was unchanged after metformin treatment (Figure 2A-B).
Metformin administration helps FA HSPCs maintain quiescence. (A) Representative flow cytometry profiles of the cell cycle analysis for KSL cells: placebo-treated Fancd2+/+ KSL cells (i), MET-treated Fancd2+/+ KSL cells (ii), placebo-treated Fancd2−/− KSL cells (iii), and MET-treated Fancd2−/− KSL cells (iv). The percentages on the profiles indicate the mean value for each group. (B) Statistical analysis of the cell cycle status. Data are poled results from 10 to 15 mice.
Metformin administration helps FA HSPCs maintain quiescence. (A) Representative flow cytometry profiles of the cell cycle analysis for KSL cells: placebo-treated Fancd2+/+ KSL cells (i), MET-treated Fancd2+/+ KSL cells (ii), placebo-treated Fancd2−/− KSL cells (iii), and MET-treated Fancd2−/− KSL cells (iv). The percentages on the profiles indicate the mean value for each group. (B) Statistical analysis of the cell cycle status. Data are poled results from 10 to 15 mice.
Next, we performed the CFU-S assay, a short-term quantitative in vivo functional assay for HSPCs, using bone marrow from metformin-treated mice (Figure 3A). Metformin-treated Fancd2−/− bone marrow cells formed almost twice as many macroscopic splenic colonies (P < .03) as placebo-treated Fancd2−/− controls (Figure 3B), suggesting a marked improvement of HSPC function in metformin-treated Fancd2−/− bone marrow. In contrast, the CFU-S forming capacity in wild-type bone marrow was unchanged after metformin treatment (P = .80; Figure 3B).
Metformin administration improves the function of FA bone marrow cells. (A) Representative pictures of spleens analyzed in the CFU-S assay. (B) Statistical analysis of CFU-S assays. Forty thousand donor bone marrow cells were injected intravenously into each recipient mouse. Data represent 3 or 4 donors in each group of mice, with 2 to 4 recipients for each donor. (C) Schematic chart to show the procedures of poly(I:C) experiments. Three-month-old mice were injected intraperitoneally with either poly(I:C) or saline at 8 mg/kg body weight. The mice were harvested either 2 weeks (for CFU-S assay) or 3 weeks (for CBC analysis) after the completion of poly(I:C) treatment. (D) Statistical analysis of CFU-S assays after poly(I:C) administration. Data represent 8 or 9 donors in each group of mice, with 2 to 4 recipients for each donor. Total recipients in each group ranged from 23 to 28 mice. (E) Statistical analysis of CBC tests after poly(I:C) administration. Data are pooled results from 11 to 17 mice each group for wild-type mice and 18 to 19 mice each group for Fancd2−/− mice.
Metformin administration improves the function of FA bone marrow cells. (A) Representative pictures of spleens analyzed in the CFU-S assay. (B) Statistical analysis of CFU-S assays. Forty thousand donor bone marrow cells were injected intravenously into each recipient mouse. Data represent 3 or 4 donors in each group of mice, with 2 to 4 recipients for each donor. (C) Schematic chart to show the procedures of poly(I:C) experiments. Three-month-old mice were injected intraperitoneally with either poly(I:C) or saline at 8 mg/kg body weight. The mice were harvested either 2 weeks (for CFU-S assay) or 3 weeks (for CBC analysis) after the completion of poly(I:C) treatment. (D) Statistical analysis of CFU-S assays after poly(I:C) administration. Data represent 8 or 9 donors in each group of mice, with 2 to 4 recipients for each donor. Total recipients in each group ranged from 23 to 28 mice. (E) Statistical analysis of CBC tests after poly(I:C) administration. Data are pooled results from 11 to 17 mice each group for wild-type mice and 18 to 19 mice each group for Fancd2−/− mice.
Metformin administration protects FA cells from poly(I:C)-induced hematologic abnormalities
To further evaluate metformin’s effects on hematopoiesis, we took advantage of the recent finding that HSC cycling induced by poly(I:C) in Fanca−/− mice caused aplastic anemia.27 As depicted in Figure 3C, cohorts of 3-month-old Fancd2−/− mice and wild-type controls on metformin or placebo diet were given 3 consecutive high doses of poly(I:C) spaced 3 days apart. The mice were harvested 2 weeks after the completion of poly(I:C) treatment followed by analyses of bone marrow function. The CFU-S forming capacity of the bone marrow in Fancd2−/− mice was dramatically reduced after poly(I:C) administration (P = .0001; Figure 3D). Importantly, Fancd2−/− mice fed with a metformin diet while being given poly(I:C) displayed a complete protection from poly(I:C)-induced loss of HSPC activity, as evidenced by their maintenance of normal levels of CFU-S forming potential (P < .01; Figure 3D). This protection may reflect the ability of metformin to reenforce HSPC quiescence and hence counteract the deleterious effects of poly(I:C)-induced cycling on HSCs.
One characteristic FA patient phenotype, red cell deficiency, only becomes apparent in very old (18 months) Fancd2−/− mice.9 However, only 3 weeks after poly(I:C) administration in 3-month-old Fancd2−/− mice, CBC analysis revealed a red cell deficiency and low hemoglobin levels (P < .01; Figure 3E). This effect was not observed in comparable control wild-type mice (P = .35). In a parallel study where Fancd2−/− mice were simultaneously fed with metformin while being given poly(I:C), metformin-fed mice displayed a clear protection from poly(I:C)-induced red cell deficiency (P < .01; Figure 3E). Collectively, these results indicate that metformin protects Fancd2−/− mice from poly(I:C)-induced hematologic abnormalities.
Metformin reduces DNA damage in FA cells
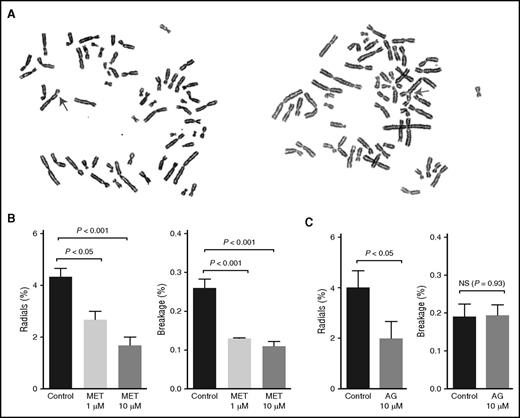
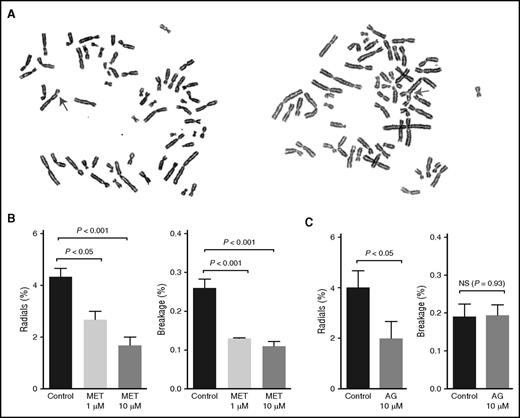
Because of a deficiency in interstrand cross-link repair, FA cells display high levels of radial chromosome formation and chromosomal breaks. These chromosomal changes are characteristic features of FA cells and are widely used to help confirm a clinical diagnosis of FA (Figure 4A).28 Treatment with DNA cross-linking agents strongly induces this phenotype, but some FA cells also display spontaneously elevated chromosome breakage levels. We thus used this classic radial and breakage assay to determine whether metformin could protect FA cells from spontaneous DNA damage. FA-A patient–derived fibroblast cells (PD259i) that displayed spontaneous radials and breakage were treated with metformin (1 µM or 10 µM) for 48 hours before cytogenetic analysis. As shown in Figure 4B, metformin significantly reduced the levels of both radials and chromosomal breaks in PD259i cells, indicating that metformin can protect FA cells from developing DNA damage.
Metformin prevents FA patient–derived cells from developing radials and chromosomal breaks. (A) Representative pictures of spontaneous radials and breaks in PD259i human FA-A fibroblasts. The arrows indicate a chromosomal break (left) or a radial (right). (B-C) Statistical quantitation of radials and breaks in PD259i human FA-A fibroblasts after aminoguanidine or metformin treatment. PD259i cells were maintained in DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin. Cells were cultured with metformin or aminoguanidine for 48 hours before metaphase spreads were made. Fifty metaphases for each sample were scored for radial contents and chromosomal breakage. Data are combined results from 6 independent experiments. AG, aminoguanidine.
Metformin prevents FA patient–derived cells from developing radials and chromosomal breaks. (A) Representative pictures of spontaneous radials and breaks in PD259i human FA-A fibroblasts. The arrows indicate a chromosomal break (left) or a radial (right). (B-C) Statistical quantitation of radials and breaks in PD259i human FA-A fibroblasts after aminoguanidine or metformin treatment. PD259i cells were maintained in DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin. Cells were cultured with metformin or aminoguanidine for 48 hours before metaphase spreads were made. Fifty metaphases for each sample were scored for radial contents and chromosomal breakage. Data are combined results from 6 independent experiments. AG, aminoguanidine.
To better understand the mechanism behind this protective effect, we also tested another guanidine derivative, aminoguanidine, in the same assay. As shown in Figure 4C, aminoguanidine also significantly suppressed the formation of radials in PD259i cells, consistent with the chemical similarity and inferred mode of action of these 2 compounds.
Metformin may act by aldehyde detoxification
Increased sensitivity to DNA cross-linking agents such as formaldehyde and mitomycin C (MMC) is a characteristic hallmark of FA cells. Recent research has emphasized the role of endogenously produced aldehydes in producing DNA interstrand cross-links and contributing to the pathogenesis of bone marrow failure in FA.10,11,13,29 In particular, endogenous formaldehyde, a highly reactive and abundant aldehyde generated by normal cellular processes such as DNA demethylation, has recently been shown to be an HSC genotoxin.12 It is known that FA cells are sensitive to formaldehyde.30 Consistent with these observations, we were able to demonstrate that cultured FA-G patient–derived lymphoblastoid cells (EUFA316) were sensitive to both the classic DNA cross-linking agent MMC and to formaldehyde (Figure 5A,C). Guanidine derivatives such as metformin and aminoguanidine have the ability to react with aldehydes through the Mannich reaction15,31 and could potentially serve as aldehyde scavengers in FA cells to prevent DNA damage. Indeed, we found that aminoguanidine protected EUFA316 cells from dose-dependent, formaldehyde-induced growth arrest (Figure 5B). Surprisingly, we also observed a mild protection of aminoguanidine from MMC-induced growth arrest (Figure 5D).
Aldehyde sensitivity of human FA cells and the detoxification of aldehydes by aminoguanidine. (A) Formaldehyde dose-dependent survival of EUFA316 human FA-G mutant lymphoblastoid cells compared with an isogenic, FANCG-complemented EUFA316 control. Complementation of patient cells was performed by stably transducing FANCG-mutant EUFA316 cells with a retrovirus expressing a wild-type human FANCG complementary DNA. EUFA316 and EUFA316+FANCG cells were cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. (B) Aminoguanidine shows dose-dependent rescue of EUFA316 cells from formaldehyde-induced cell death. (C) MMC dose-dependent survival of EUFA316 and wild-type controls. (D) Aminoguanidine provided a mild protection on EUFA316 cells from MMC-induced cell death. (E-F) Statistical quantitation of radials and chromosomal breaks in 259i human FA-A fibroblasts treated with C3, the ADH5 inhibitor. Metformin was added to the cell culture at 10 µM and maintained at the same concentration throughout the experiment. One hour later, C3 was added at 100 µM. Forty-eight hours later, cells were harvested for breakage and radial analysis. Data are combined results from 4 independent experiments.
Aldehyde sensitivity of human FA cells and the detoxification of aldehydes by aminoguanidine. (A) Formaldehyde dose-dependent survival of EUFA316 human FA-G mutant lymphoblastoid cells compared with an isogenic, FANCG-complemented EUFA316 control. Complementation of patient cells was performed by stably transducing FANCG-mutant EUFA316 cells with a retrovirus expressing a wild-type human FANCG complementary DNA. EUFA316 and EUFA316+FANCG cells were cultivated in RPMI 1640 medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. (B) Aminoguanidine shows dose-dependent rescue of EUFA316 cells from formaldehyde-induced cell death. (C) MMC dose-dependent survival of EUFA316 and wild-type controls. (D) Aminoguanidine provided a mild protection on EUFA316 cells from MMC-induced cell death. (E-F) Statistical quantitation of radials and chromosomal breaks in 259i human FA-A fibroblasts treated with C3, the ADH5 inhibitor. Metformin was added to the cell culture at 10 µM and maintained at the same concentration throughout the experiment. One hour later, C3 was added at 100 µM. Forty-eight hours later, cells were harvested for breakage and radial analysis. Data are combined results from 4 independent experiments.
To further assess whether metformin or aminoguanidine could protect FA-deficient cells, we devised a more sensitive cocultivation experiment in which equal numbers of EUFA316 cells and EUFA316+FANCG cells (stably complemented with a wild-type FANCG complementary DNA–encoding retrovirus) were labeled with different fluorescent proteins and allowed to compete in the presence of media only, 40 µM formaldehyde, or 6.25 nM MMC together with metformin or aminoguanidine (0.01, 0.1, or 1 mM). The competitive growth of these cells was monitored by flow cytometry. Both aminoguanidine and, to a lesser degree, metformin were able to provide dose-dependent protection against exogenous formaldehyde (supplemental Figure 1). We also observed protection against MMC by both aminoguanidine and metformin, although only at the highest dose tested (1 mM; supplemental Figure 1), probably reflecting the nonspecific protective effects of suppressed cell cycling by aminoguanidine or metformin. These results demonstrate that metformin and aminoguanidine protect FA-deficient cells from formaldehyde-induced and, to a much lesser extent, MMC-induced toxicity.
Formaldehyde is detoxified principally by formaldehyde dehydrogenase, encoded by the Adh5/Gsnor gene. Adh5−/− mice accumulate formaldehyde adducts in DNA.12 To determine whether metformin was able to suppress formaldehyde-induced DNA damage, we used a recently discovered small-molecule inhibitor of Adh5, the C3 compound, to induce the accumulation of endogenous formaldehyde in FA cells.32,33 After human FA-A patient–derived fibroblast cells PD259i were treated with 100 µM C3 for 48 hours, the levels of spontaneous radials and chromosomal breaks were significantly increased by twofold and threefold, respectively (P < .0001 in both cases; Figure 5E-F). Importantly, concurrent treatment with metformin significantly suppressed C3-induced radials and chromosomal breaks (P < .0001 and P < .001, respectively; Figure 5E-F), consistent with a role for metformin in detoxifying formaldehyde.
Metformin delays tumor formation in Fancd2−/− mice
The in vitro experiments above showed that metformin could reduce spontaneous DNA damage in FA cells. In addition, metformin is well known to reduce the incidence of several human cancers.34,35 For these reasons, metformin was tested as a cancer chemopreventive agent. A cohort of tumor prone Fancd2−/−Trp53+/− mice along with Fancd2+/+Trp53+/− littermate controls were divided into 2 groups and treated with either metformin or placebo diet. The tumor spectrum in metformin-treated Fancd2−/−Trp53+/− mice was similar to that in placebo-treated controls. The most common type of tumor was ovarian in origin, consistent with our previous observation on tumor types in Fancd2−/− mice9,22 and an earlier study reporting that more than 18% of human primary ovarian epithelial cancers have a disrupted FA/BRCA pathway.36 Specifically, 61 Fancd2−/−Trp53+/− mice under placebo treatment developed 91 tumors, among which 37 (41%) were ovarian tumors; 31 Fancd2−/−Trp53+/− mice under metformin treatment developed 34 tumors, among which 13 (38%) were ovarian tumors. These spectra were similar to what we have reported before.37 However, as shown in Figure 6, Fancd2−/−Trp53+/− mice on metformin diet showed a significantly longer (P < .05) mean tumor-free survival time (mean survival of 405 days) than the Fancd2−/−Trp53+/− mice on placebo diet (mean survival of 368 days). The first tumor was seen at 142 days in the Fancd2−/−Trp53+/− mice on placebo diet, whereas the earliest tumor in the Fancd2−/−Trp53+/− mice on metformin appeared much later at 244 days of age. Overall, these results indicate that metformin administration significantly delays tumor formation in Fancd2−/−Trp53+/− mice.
Metformin protects Fancd2−/− mice from tumor development. Kaplan-Meier survival curves of the Fancd2−/−Trp53+/− mice and Fancd2+/+Trp53+/− mice. For Fancd2−/−Trp53+/− mice, the data represent 31 mice for metformin treatment and 60 mice for placebo treatment. For Fancd2+/+Trp53+/− mice, the data represent 30 mice for metformin treatment and 60 mice for placebo treatment. Tumor samples and selected tissues were fixed in 10% phosphate-buffered formalin, stained with hematoxylin and eosin, and examined under a microscope. The Kaplan-Meier survival curves were generated by Prism 6.0c Software (GraphPad Software, Inc.), and P values were calculated using the log-rank (Mantel-Cox) test.
Metformin protects Fancd2−/− mice from tumor development. Kaplan-Meier survival curves of the Fancd2−/−Trp53+/− mice and Fancd2+/+Trp53+/− mice. For Fancd2−/−Trp53+/− mice, the data represent 31 mice for metformin treatment and 60 mice for placebo treatment. For Fancd2+/+Trp53+/− mice, the data represent 30 mice for metformin treatment and 60 mice for placebo treatment. Tumor samples and selected tissues were fixed in 10% phosphate-buffered formalin, stained with hematoxylin and eosin, and examined under a microscope. The Kaplan-Meier survival curves were generated by Prism 6.0c Software (GraphPad Software, Inc.), and P values were calculated using the log-rank (Mantel-Cox) test.
In contrast, as shown in Figure 6, the Fancd2+/+Trp53+/− mice on placebo diet had a mean tumor-free survival of 510 days, and those on metformin diet survived an average of 535 days. There was no significant difference between metformin and placebo treatment in these p53 heterozygotes (P = .86), indicating that the tumor-delaying effect of metformin was specific to only FA mutant mice.
Discussion
The majority of genes responsible for FA have now been found and many of their biochemical functions are being uncovered.2,3 The FA network consists of at least 21 proteins that serve to maintain genome stability, enhance stem cell survival, and suppress cancer and are functionally integrated with genes involved in inherited breast and ovarian cancers (eg, BRCA1 and BRCA2). Despite these scientific insights, there has been little progress in treating human FA patients or preventing bone marrow failure. Bone marrow transplantation is currently the only curative therapy for the hematopoietic complications of the disorder, but when performed without a matched sibling donor, it is often accompanied by both short-term and long-term morbidities.1 Among these complications is a very high risk of secondary cancer.38 Synthetic androgens have been used for many years to support marrow function and improve cytopenias for a subset of FA patients.39,40 However, these outcomes are limited by incomplete or transient responses, together with unacceptable side effects and toxicities. Gene therapy remains a promising approach for FA,41 but to date there have been no reports of clinical success despite the selective advantage for gene corrected stem cells in this disorder. Furthermore, as noted previously, successful treatment of bone marrow failure does not diminish the severity or risk of nonhematopoietic consequences of FA, most notably the high risk of solid tumors.38 New therapeutic approaches that have the ability to treat or prevent bone marrow failure and cancer are thus clearly needed for FA.8,9,20,37,42
Here we show that the widely used diabetes drug metformin improves hematopoiesis and delays tumor formation in Fancd2−/− mutant mice. Of note, metformin is the first compound to improve both of these FA phenotypes: oxymetholone,9 resveratrol,8 sirtuin activator,20 and N-acetylcysteine42 all improve hematopoiesis in Fancd2−/− mice, but none has been shown to diminish tumor incidence. In contrast, the antioxidant tempol delays cancer in FA but does not benefit hematopoiesis.37 Our results indicate that metformin can ameliorate both of these key phenotypes of FA, and that its beneficial action was specific to FA mutant mice. In contrast, oxymetholone and the sirtuin activator SRT3025 affect both wild-type and mutant stem cells equally,9,20 indicating that their mechanisms of action do not target the pathophysiology of FA bone marrow failure. Furthermore, it is particularly intriguing that the chronic administration of metformin significantly increases the frequency of HSCs in the adult Fancd2−/− mice. The loss of HSCs in FA lies at the root of bone marrow failure and is a progressive process that extends from adolescence into adulthood.5,6,9 There is an emerging body of evidence supporting that this progressive HSC loss may begin in utero.8,43,44 Although the magnitude of HSC rescue by metformin is relatively small, this drug demonstrates a unique ability to restore the HSC numbers in postnatal life in FA mice.
The specificity of action of metformin and the structurally related compound aminoguanidine in the protection of FA mutant cells may be explained by our observation that both compounds appear to selectively reduce DNA damage in FA cells. This is demonstrated by the dose-dependent reduction of spontaneous radial chromosome formation and chromosome breaks in a human FA cell line treated with either compound.
The precise mechanism by which metformin and aminoguanidine reduce DNA damage in FA cells remains unclear, although here we present evidence that aldehyde detoxification may be an important part of the protective effect conferred by both metformin and aminoguanidine. Endogenously produced aldehydes, including acetaldehyde and formaldehyde, are clearly genotoxic in FA mutant cells.11,12,29,45 We found that the pharmacological inhibition of Adh5, the main enzyme responsible for cellular formaldehyde detoxification, induced chromosome breakage and radials in FA cells. Importantly, metformin rescued this defect at physiologically relevant concentrations. Given that the chemically related guanidine derivative aminoguanidine was also able to block formaldehyde toxicity, presumably through the well-described Mannich reaction,15,31 our data are consistent with the hypothesis that metformin acts at least in part through an aldehyde scavenging mechanism. It is surprising that metformin also mildly protected FA patient cells from MMC-induced growth arrest. This could be because of the release of lipid peroxidation–derived aldehyde 4-hydroxy-2-nonenal associated with MMC treatment46 and/or methanol (which could be oxidized to formaldehyde) during the activation of MMC.47 However, it is also possible that metformin may act via other mechanisms to attenuate the FA phenotype. For example, metformin potently activates AMPK, a kinase known to be important in protecting HSCs from genomic instability.21 Of note, in our previous transcriptome analysis of HSPCs,9 the messenger RNA encoding SLC22A3, one of the membrane transporters important for metformin uptake,34,48 is preferentially enriched by 12.3-fold in HSPCs, as compared with whole bone marrow cells. It is thus possible that metformin can exert its effects directly on HSPCs. Metformin’s effects on the Fancd2−/− hematopoietic system, including reenforcing quiescence in HSPCs and increasing CFU-S–forming capacity of bone marrow cells, resemble the effects of resveratrol,8 another known AMPK activator.19 It is, therefore, tempting to suggest that metformin and resveratrol might exert their hematopoietic benefits through the AMPK signaling pathway. However, this does not explain all the effects from metformin because metformin delays tumor formation in Fancd2−/− mice, whereas resveratrol does not. We recently discovered that transforming growth factor β (TGF-β) inhibitors can protect HSCs in FA mice and patients by altering the balance of nonhomologous end joining and homologous recombination.49 Although metformin is not known to directly interfere with TGF-β signaling, several reports indicate interactions between the LKB1/AMPK pathways and TGF-β.50 Other mechanisms by which metformin may act to protect FA mice include the following: reducing the activity of mitochondrial complex 1 activity,14 thus potentially reducing oxidative DNA damage51,52 ; supporting the expansion of HSCs in vitro by switching the metabolic balance between oxidative phosphorylation and anaerobic glycolysis53 ; and downregulating inflammatory pathways,54 which are thought to contribute to bone marrow failure in FA.55 Future studies will be needed to determine whether 1 or more of these known mechanisms in addition to aldehyde quenching are responsible for the beneficial effects of metformin in FA.
Despite these uncertainties as to the exact mechanism of protection by metformin, a compelling argument can be made for a clinical trial of metformin to protect FA patients from bone marrow failure and tumorigenesis. Metformin has a superb safety record in light of its wide clinical use to treat diabetes mellitus over >2 decades. In our preclinical model, metformin outperforms the current standard of care, oxymetholone. Oxymetholone therapy had no significant effect on peripheral blood counts of FA mice at 6 months.20 Its benefits only became apparent after 17 months of treatment.9 In contrast, metformin improved peripheral blood counts after only 6 months of therapy.
Several important questions remain to be answered. The optimal dose of metformin for FA therapy and disease prevention is not known. If metformin acts predominantly as an aldehyde scavenger, higher doses may be optimal, and a well-tolerated high dose could be readily determined from use and toxicity data. It is also not known when beginning metformin treatment would be most beneficial: prior to the onset of bone marrow failure or only after the development of anemia. Finally, potential synergies between metformin and anabolic androgens, the current gold standard of therapy, have not been studied. These interactions are difficult to predict directly, as androgens accelerate the cell cycle of stem cells,9 whereas metformin increases quiescence.
Our results may also have relevance in regard to the use of metformin in the general population as an antiaging and cancer chemoprevention drug.56 Metformin has not previously been reported to protect the genome from DNA damage and mutation. However, such activity would go a long way toward explaining why it can.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Pamela Canady and Mandy Boyd at the Oregon Health & Science University flow cytometry core for fluorescence-activated cell sorting, William H. Fleming and Devorah C. Goldman for valuable advice, and Laura Marquez-Loza for help with animal care.
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health National Heart, Lung, and Blood Institute (grant P01 HL048546) (M.G.), the Fanconi Anemia Research Foundation (M.G.), and an award from the Office of the Assistant Secretary of Defense for Health Affairs, through the Bone Marrow Failure Research Program (award no. W81XWH-14-1-0297) (R.J.M.).
Authorship
Contribution: Q.-S.Z. designed the study, performed research, analyzed and interpreted the data, and wrote the manuscript; M.D., N.P., W.T., H.L., M.A.-D., and A.M. performed research; R.J.M. and S.O. designed the in vitro studies, interpreted the data, and wrote the manuscript; A.N.M. examined the histological slides and wrote the manuscript; and M.G. designed the study, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.L. is Department of Anatomy and Neurobiology, Northeastern Ohio Medical University, Rootstown, OH.
Correspondence: Qing-Shuo Zhang, Oregon Stem Cell Center, Department of Pediatrics, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; e-mail: zhangqi@ohsu.edu.