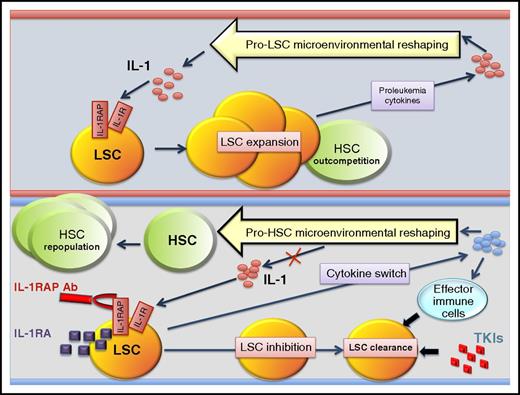
In this issue of Blood, Ågerstam et al1 and Zhang et al2 show that increased expression of the interleukin-1 receptor (IL-1R) complex confers leukemia stem cells (LSCs) from chronic myeloid leukemia (CML) with a selective growth and survival advantage over normal hematopoietic stem cells (HSCs).1,2 Furthermore, they provide evidence that blocking IL-1 signaling through IL-1 receptor antagonist (IL-1RA)2 or antibody-mediated therapy (anti-IL-1R accessory protein [anti-IL-1RAP])1 has the potential to lead to disease eradication at a stem cell level (see figure). This is extremely important because first-, second-, and third-generation BCR-ABL1 TKIs, although they are capable of inducing sustained molecular and deep molecular remission, are unable to kill quiescent LSCs. Thus, it is becoming evident that a better characterization of the specific pathways of LSCs could lead to the development of new therapies that, if not toxic, will selectively kill CML LSCs. The therapeutic relevance of such studies rests on the fact that a successful CML eradication therapy will not only prevent disease relapse upon TKI discontinuation but also release the majority of patients from life-long and economically challenging TKI therapy and, perhaps, will positively impact prognosis of those patients who become resistant to multiple TKIs and/or undergo blastic transformation.3
LSCs but not HSCs exploit IL-1 signaling in the CML BM microenvironment. Increased IL-1 levels and IL-1R1 and IL-1RAP overexpression confer a selective advantage on LSCs over HSCs in the leukemic BM microenvironment. Targeting of IL-1 signaling via antibody and antagonist (IL-1RA) therapy eliminates the proliferative advantage, reshapes the microenvironment, and favors elimination of LSCs by effector cells and TKIs. Ab, antibody; TKIs, tyrosine kinase inhibitors.
LSCs but not HSCs exploit IL-1 signaling in the CML BM microenvironment. Increased IL-1 levels and IL-1R1 and IL-1RAP overexpression confer a selective advantage on LSCs over HSCs in the leukemic BM microenvironment. Targeting of IL-1 signaling via antibody and antagonist (IL-1RA) therapy eliminates the proliferative advantage, reshapes the microenvironment, and favors elimination of LSCs by effector cells and TKIs. Ab, antibody; TKIs, tyrosine kinase inhibitors.
Recently, a few stem cell markers have been described as potential targets for a biological cure for CML. Among these, IL-1RAP is selectively expressed in CML LSCs and not in HSCs from healthy individuals. Induction of IL-1RAP seems to be dependent on BCR-ABL1 kinase in progenitors but not in LSCs, and targeting it with antibodies resulted in a decreased number of CML stem cells.1,4 This is not unique because other LSC markers such as CD26 and CD123 hold great therapeutic promise as inhibitors of their signaling, and antibodies capable of inducing antibody-dependent cellular cytotoxicity (ADCC) have been successfully tested in preclinical settings.5,6 However, the striking ability of LSCs to evade therapy by generating and exploiting specific microenvironmental signals poses potential obstacles to the success of old and new strategies.7-9
In this context, a precise analysis of the bone marrow (BM) milieu that selectively supports the expansion of LSCs may highlight new ways to increase efficacy of available therapies and/or the development of new ones. Indeed, CML LSCs and progenitors induce striking changes in the BM (eg, tissue fibrosis) thereby creating a niche in which extrinsic factors (eg, cytokines) favor the engraftment, expansion, and survival of LSCs over HSCs.8,9 Further proof of the peculiar ability of LSCs to exploit a specific leukemia-induced cytokine milieu is provided by the observation that mouse leukemic long-term repopulating HSCs (LT-HSCs) gain a proliferative advantage over their normal counterparts when cultured in conditioned medium from CML cells but not from normal cells.7
Here, Ågerstam et al and Zhang et al show that both IL-1 availability and its selective exploitation by LSCs play a significant role in the growth, survival, and drug resistance of LSCs. In fact, mitogenic and survival signals driven by nuclear factor κB (NF-κB), p38MAPK, and JNK pathways are markedly increased in IL-1–stimulated CD34+CD38–CD90+ through triggering of IL-1R and IL-1RAP.1,2 Thus, it is possible that the increased levels of IL-1 in the CML BM microenvironment7 can be exploited by LSCs to gain a selective advantage over HSCs. Indeed, both research groups reported a selective response of LSCs, but not their healthy counterparts, to inhibition of IL-1 signaling.1,2 In this regard, IL-1RAP knockout mice have normal hematopoiesis.1 Likewise, BM engraftment was similar in competitive repopulating assays with IL-1RAP wild-type and knockout HSCs, suggesting that the IL-1 signaling pathway is not a requirement for the maintenance and development of nontransformed HSCs.1
Accordingly, HSC number and/or activity were not altered upon exposure to the IL-1 signaling antagonist IL-1RA that, instead, significantly inhibited growth and increased TKI (ie, nilotinib) sensitivity of mouse BCR-ABL1+ LT-HSCs and human CML LSC cultures in the presence of CML BM–conditioned medium.2 Similarly, in vitro exposure to IL-1RAP antibodies decreased NF-κB and Akt activity in CD34+CD38– CML cells.1 Furthermore, despite a correct in vivo evaluation that the dependence of human LSCs on IL-1 signaling (eg, NF-κB and Akt) is hindered by the poor responsiveness of these cells to mouse IL-1,1 treatment with IL-1RA was still able to significantly increase TKI-induced clearance of LSCs in CML-engrafted mice.2
In addition to providing evidence that IL-1RA may potentially be used in the clinic for inducing the killing of LSCs, the data presented in this issue of Blood offer an additional therapeutic strategy. In fact, the use of IL-1RAP antibodies resulted in ADCC-dependent increased survival of mice engrafted with primary human CML and BV173 (lymphoid blast crisis [BC]) cells,1 suggesting that blocking IL-1 signaling may benefit CML-BC patients, too. In fact, increased IL-1R type 1 (IL-1R1) and IL-1RAP expression and IL-1–dependent NF-κB induction were also observed in CD34+CD38+ CML-BC progenitors,1 suggesting that targeting IL-1 signaling in CML-BC might simultaneously lead to apoptosis of both CML LSCs and CML progenitors with or without leukemia-initiating capacity.10
In conclusion, the data presented in this issue of Blood demonstrate that eradication of LSCs can be achieved by using IL-1RAP to direct ADCC1 or by blocking IL-1 signaling,1,2 thereby exploiting both surface molecules and signaling characteristics unique to LSCs. In addition, because cytokine secretion is altered after IL-1RA treatment,2 targeting the IL-1 receptor may prevent the generation of BM extrinsic signals that contribute to the persistence of LSCs in TKI-treated patients. Finally, inhibition of IL-1 signaling significantly impaired survival of BCR-ABL1+ CML-BC progenitors both in vitro and in vivo, suggesting that such a therapeutic approach may also benefit CML-BC patients who do not show long-term response to TKI monotherapy.3
Conflict-of-interest disclosure: The authors declare no competing financial interests.