Abstract
Background: Chronic GVHD (cGVHD) is a serious complication of allogeneic stem cell transplantation. Effective therapy for patients (pts) with cGVHD who fail corticosteroids remains an unmet medical need. Both B and T cells play a role in the pathophysiology of cGVHD. In preclinical models, ibrutinib (ibr) reduced the severity of cGVHD through its inhibition of Bruton's tyrosine kinase (BTK) and interleukin-2-inducible T-cell kinase (ITK) (Dubovsky, J Clin Invest 2014). Ibr has recently been granted a breakthrough therapy designation (BTD) for cGVHD after failure of 1 or more lines of systemic therapy. The request for a BTD was supported by early data from a phase 2 study that evaluated the efficacy and safety of ibr in pts with cGVHD who are in need of additional therapy. The final results of this same study are presented here.
Methods: Pts who had received ≤3 prior regimens for cGVHD and had either >25% body surface area erythematous rash or a National Institutes of Health (NIH) mouth score >4 were treated with daily ibr (420 mg) until cGVHD progression or unacceptable toxicity. The primary end point was cGVHD response based on the 2005 NIH consensus response criteria. Secondary end points included rate of sustained response, change in Lee cGVHD symptom scale, changes in corticosteroid requirement over time, and safety end points. The effects of ibr on lymphoid and myeloid cell signaling pathways, and phenotype along with plasma cytokines and chemokines were evaluated.
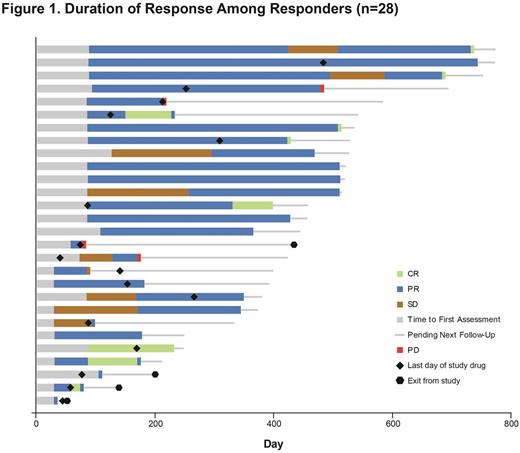
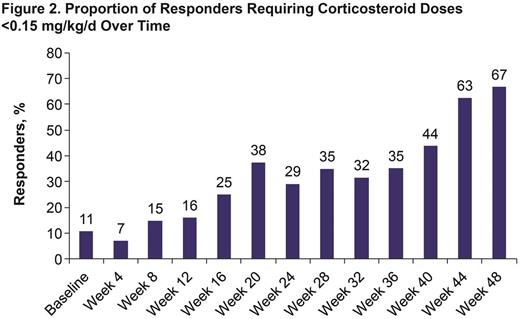
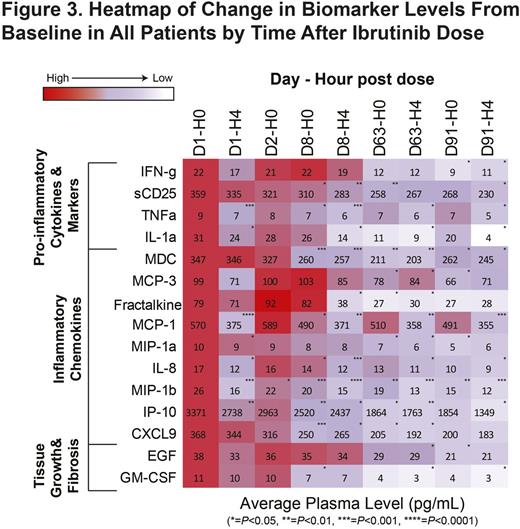
Results: A recommended phase-2 dose of 420 mg was identified in phase 1b (n=6) with no dose-limiting toxicities reported. Of 42 pts treated with ibr, median age was 56 y (range, 19-74). Median duration of cGVHD before study entry was 13.7 mo (range, 1.1-63.2). Median number of prior regimens was 2 (range, 1-3). At a median follow-up of 13.9 mo, overall response rate (ORR) was 67% (28/42 pts; 9 [21%] CR, 19 [45%] PR), with 20/28 (71%) and 12/25 (48%) responders showing a sustained response of ≥20 and ≥32 weeks, respectively (Figure 1). Overall, 21 responders (75%) had corticosteroid doses <0.15 mg/kg/d during the study (Figure 2). Five responders discontinued corticosteroid therapy while in response. Median overall clinician-assessed cGVHD severity score decreased from 7 at baseline (n=41) to 4 at week 25 (n=20) and 3 at week 49 (n=15). A corresponding decrease in median pt-assessed overall cGVHD score from 7 at baseline (n=42) to 5 at week 25 (n=18) and 4 at week 49 (n=14) was reported. Improvement in Lee cGVHD symptom score was reported for 12 (43%) and 17 (61%) of 28 responders by month 6 and overall, respectively, compared with 1 (11%) of 9 nonresponders by month 6 and overall. Of 36 pts with ≥2 involved organs, 20 (56%) showed a response in ≥2 organs; of 12 pts with ≥3 involved organs, 5 (42%) showed a response in ≥3 organs. Analysis of soluble plasma factors associated with inflammation, fibrosis, and cGVHD from all treated pts showed a significant decrease over time with ibr, including soluble CD25 and IP-10 levels, which are implicated in cGVHD-related inflammation (Figure 3). The most common adverse events (AEs) were fatigue (57%), diarrhea (36%), muscle spasms (29%), nausea (26%), and bruising (24%). Grade ≥3 AEs occurring in >3 pts were pneumonia (n=6), fatigue (n=5), and diarrhea (n=4). Serious AEs (SAEs) occurred in 22 pts (52%); grade ≥3 SAEs reported in 17 pts (40%) included pneumonia (n=5), septic shock (n=2), and pyrexia (n=2). Two fatal events (multilobular pneumonia and bronchopulmonary aspergillosis) were reported. Five pts discontinued therapy for progressive cGVHD and 14 for AEs including fatigue (n=3) and pneumonia (n=2). Twelve pts (29%) continued ibr; their treatment duration ranges from 5.6-24.9 mo.
Conclusions: With an ORR of 67% and a sustained response rate of ≥20 weeks of 71%, treatment with ibr resulted in clinically meaningful and durable responses in pts who failed at least 1 prior treatment for cGVHD. Most responders were able to reduce steroid dose to an acceptable minimal level. Biomarker changes support an effect of ibr on immune cell subsets in pts with cGVHD. Reported AEs are consistent with those for ibr in B-cell malignancies and pts with cGVHD. Observed response rates in this pretreated, high-risk population support further study of ibr for frontline treatment of cGVHD.
Miklos:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Other: Travel, Accommodations, and Expenses, Research Funding; Kite Pharma: Research Funding; Roche: Research Funding; Novartis: Research Funding; Sanofi Oncology: Other: Travel, Accommodations, and Expenses. Cutler:Insys: Consultancy; Seattle Genetics: Consultancy; Regimmunie: Consultancy; Incyte: Consultancy. Arora:Takeda Oncology: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Research Funding. Waller:Cerus: Equity Ownership; Chimerix: Equity Ownership; Cambium Medical Technologies: Equity Ownership, Other: Other relationship indicated, Patents & Royalties; Novartis: Consultancy, Honoraria, Research Funding; Helocyte: Consultancy. Jagasia:Theracos: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Flowers:Pharmacyclics, LLC, an AbbVie Company: Research Funding. Logan:Amgen: Consultancy; Jazz: Consultancy; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding; Astellas: Research Funding; Novartis: Research Funding. Nakamura:Seattle Genetics: Consultancy; Amgen: Consultancy. Blazar:Tobira Therapeutics: Consultancy; Vulcan CApital: Consultancy; Idera Pharma: Consultancy; Sidley Austin LLP: Consultancy; Merck Serono: Consultancy; Fate Therapeutics: Consultancy; Merck, Sharpe & Dohme Corp: Consultancy; Bristol-Myers Squibb: Consultancy; Kadmon Pharmaceuticals Inc: Consultancy, Research Funding; Kymab: Consultancy; Five Prime Therapeutics: Consultancy; Vitae Pharmaceuticals, Inc.: Consultancy; Flx Bio: Consultancy; No Company: Patents & Royalties: no company. Li:Pharmacyclics, LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Lal:AbbVie: Equity Ownership; Pharmacyclics, LLC, an AbbVie Company: Employment; Infinity: Equity Ownership; Clovis: Equity Ownership; Reviva Pharmaceuticals: Equity Ownership; The Permanente Medical Group: Employment, Equity Ownership; Gilead Sciences: Employment, Equity Ownership. Dubovsky:Pharmacyclics, LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. James:Pharmacyclics, LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Styles:Pharmacyclics, LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Jaglowski:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.