Abstract
Background: Follicular lymphoma (FL) is the most common subtype of indolent non Hodgkin's lymphoma (NHL). Median survival is long (>10 years), but current chemo-immunotherapy regimens used for FL are usually not curative. While T cells in the FL tumor microenvironment are considered dysfunctional and associated with disease progression, a better understanding of T-cell signaling may reveal how to productively engage tumor-infiltrating T cells to kill lymphoma B cells. Our previous study showed that expression of the immune checkpoint receptor PD-1 was directly correlated with reduced cytokine signaling in FL T cells (Myklebust et al., Blood 2013). Antibody immunotherapy targeting the PD-1/PD-L1 pathway has shown significant activity in solid tumors, but these benefits have not been as profound in NHLs, including FL. Co-blockade of checkpoint inhibitors may therefore be necessary to generate optimal anti-tumor responses in FL. The hypothesis underlying this study was that characterizing signaling responses in FL tumor-infiltrating T cells will identify new targets for combination of checkpoint blockade.
Methods: Surface expression of 9 checkpoint receptors governing T cell function was measured in subsets of CD4 and CD8 T cells from FL lymph node tumors (n = 14) and from healthy donor tonsils (n= 11) and peripheral blood samples (n = 7) using fluorescence flow cytometry. Patterns of checkpoint receptor expression were compared with 1) intracellular phospho-protein signaling response and 2) cytokine production for subsets of T cells infiltrating FL tumors and the corresponding T-cell populations in healthy tonsils. Phospho-specific flow cytometry measured phosphorylation of STATs and T cell receptor (TCR) signaling effectors within minutes following stimulation by IL-4, IL-7, IL-21, or α-CD3+α-CD28 (TCR stimulation) antibodies.
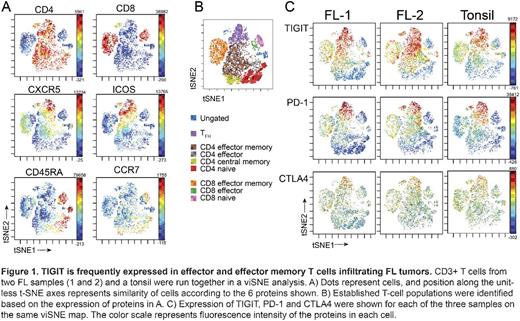
Results: CD4 and CD8 T cells infiltrating FL tumors were gated into subsets defined by PD-1 and ICOS protein expression, and compared to cognate T cell subsets in healthy tonsils. FL and tonsil T cells closely matched in their signaling responses to IL-4, IL-7, and IL-21 stimulation, with PD-1 expressing cells (CD4+PD-1hiICOS+ (TFH) and CD8+PD-1int T cells) exhibiting modest phospho-protein signaling responses compared to T cells not expressing PD-1. Furthermore, TCR membrane proximal signaling events (p-CD3ζ, p-SLP76) following TCR stimulation were comparable in FL and tonsil T cells. This contrasted reduced phospho-ERK signaling in all CD4 and CD8 T cell subsets infiltrating FL tumors which distinguished them from tonsillar T cells. IFN-γ production also differed between FL and tonsils, as CD8 T cells infiltrating FL tumors produced less IFN-γ. Reduced IFN-γ production was independent of PD-1 expression, suggesting suppressed function in these T cells which could be due to inhibitory receptors other than PD-1. Of the 9 checkpoint receptors measured, PD-1 and T cell Ig and ITIM domain (TIGIT) were expressed at the highest frequency. In FL, TIGIT was expressed in 58% and 80% of CD8 effector and effector memory cells, respectively, as compared to 43% and 68% of the cognate healthy tonsillar subsets. TIGIT was also frequently expressed in CD4 FL T cells, as 52% and 79% of effector and effector memory cells expressed TIGIT, respectively, as compared to 16% and 59% of the corresponding subsets from healthy tonsils. viSNE analysis demonstrated that TIGIT and PD-1 were frequently co-expressed in FL T cells, and a large fraction of PD-1int T cells had high expression of TIGIT (Figure 1). These results provide a rationale for co-blockade of PD-1 and TIGIT in FL and for investigation of how co-blockade impacts T cell functions.
Conclusions: These results reveal specific suppression of cytokine signaling in CD8 effector T cells infiltrating FL tumors and identify TIGIT and PD-1 as strong candidates for co-checkpoint blockade in FL. A deeper understanding of the interplay between checkpoint receptors and key T cell cytokine signaling events in FL will further assist in engineering immuno-therapeutic regiments that improve FL patient clinical outcomes.
Kolstad:Nordic Nanovector: Other: Membership of Scientific Advisory Board. Levy:Kite Pharma: Consultancy; Five Prime Therapeutics: Consultancy; Innate Pharma: Consultancy; Beigene: Consultancy; Corvus: Consultancy; Dynavax: Research Funding; Pharmacyclics: Research Funding. Irish:Incyte: Research Funding; Janssen: Research Funding; Cytobank, Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.