Abstract
Background: Treatment options for patients (pts) with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL) are limited. GADOLIN (NCT01059630) is an open-label, randomized, Phase 3 trial comparing the efficacy and safety of obinutuzumab (GA101; GAZYVA/GAZYVARO; G) plus bendamustine (B) induction, followed by G maintenance (G-B arm), with B induction (standard of care) in rituximab-refractory iNHL pts. In the primary analysis, which involved all pts enrolled as of September 1, 2014 (n=396; median observation time, 21.0 months [mo]), median Independent Review Committee (IRC)-assessed progression-free survival (PFS; primary endpoint) was longer in the G-B arm (194 pts; median not reached) than in the B arm (202 pts; 14.9 mo), with a 45% reduction in risk of progression or death (HR 0.55; 95% CI 0.40, 0.74; p=0.0001). Investigator (INV)-assessed PFS was also significantly longer in the G-B arm, but overall survival (OS) data were immature. Safety profiles were comparable. The most common grade ≥3 adverse events (AEs) were neutropenia, thrombocytopenia, anemia, and infusion-related reactions (IRRs). Seventeen additional pts were enrolled after the data cut-off for the primary analysis. Here, we report updated time-to-event and safety results from a planned analysis of all pts (n=413) using a data cut-off of April 1, 2016.
Methods: Enrolled pts were aged ≥18 years (yrs) with documented rituximab-refractory iNHL and an ECOG performance status of 0-2. Pts received either G 1000mg i.v. (days [D] 1, 8, and 15 of cycle [C] 1, and D1 of C2-6) plus B 90mg/m2/day i.v. (D1 and 2 of C1-6), or B monotherapy (120mg/m2/day i.v., D1 and 2 of C1-6); each cycle was 28 days. Following induction, pts in the G-B arm without evidence of progression received G maintenance (1000mg i.v. every 2 mo for 2 yrs or until disease progression, whichever occurred first). In the current analysis, assessments included INV-assessed PFS, OS, time to new anti-lymphoma treatment (TTNT), and safety. Efficacy assessment was performed on the intent-to-treat (ITT) population. Safety analysis included all pts who received any study treatment, excluding 2 pts who crossed over to G-B during maintenance.
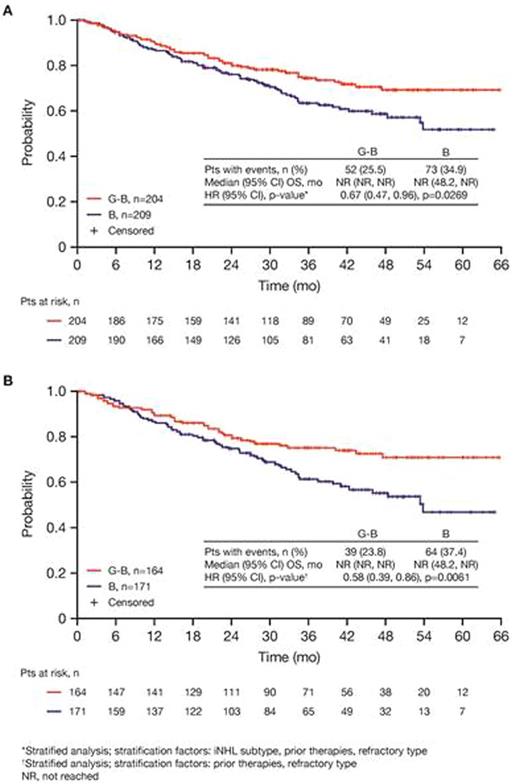
Results: Of 413 iNHL pts in the ITT population (G-B, 204; B, 209), 335 (G-B, 164; B, 171) had FL. Baseline characteristics of the ITT population were balanced between arms. Median number of prior regimens was 2 in both arms (pts with ≤2 prior regimens: G-B, 80.4%; B, 77.5%). Most pts were refractory to their last regimen (G-B, 92.2%; B, 92.3%). After 31.8 mo median follow-up, median INV-assessed PFS was 25.8 mo in the G-B arm and 14.1 mo in the B arm; HR was 0.57 (95% CI 0.44, 0.73; p<0.0001), i.e. a 43% reduction in risk of progression or death for G-B relative to B. Fewer pts died in the G-B arm (25.5%) than the B arm (34.9%), with a HR for OS of 0.67 (95% CI 0.47, 0.96; p=0.0269; risk reduction, 33%; Figure 1A); median OS was not reached for either arm. Results for FL pts were consistent with those for ITT pts (median PFS: 25.3 vs. 14.0 mo [HR 0.52; 95% CI 0.39, 0.69; p<0.0001]; median OS: not reached vs. 53.9 mo [HR 0.58; 95% CI 0.39, 0.86; p=0.0061; Figure 1B]). Median TTNT was also longer in the G-B arm than in the B arm (ITT pts: 40.8 vs. 19.4 mo, respectively [HR 0.59; 95% CI 0.45, 0.77]; FL pts: 33.6 vs. 18.0 mo, respectively [HR 0.57; 95% CI 0.43, 0.75]). The overall safety profile of G-B remained consistent with results of the primary analysis. In the ITT population, there were more grade ≥3 AEs with G-B than with B (72.5% vs. 65.5%, respectively), notably neutropenia (34.8% vs. 27.1%) and IRRs (9.3% vs. 3.5%); grade ≥3 thrombocytopenia (10.8% vs. 15.8%) and anemia (7.4% vs. 10.8%) were less frequent in the G-B arm, while grade ≥3 infections (22.5 % vs. 19.2%) and secondary malignancies (5.9% vs. 5.4%) were reported with a similar incidence. Serious AEs were more frequent in the G-B arm (43.6% vs. 36.9%), but the incidence of grade 5 (fatal) AEs was similar (7.8% vs. 6.4%). Safety results in FL pts were comparable with those in all iNHL pts.
Conclusions: Updated analysis of the GADOLIN study with ~10 mo additional follow-up confirms the previously reported PFS benefit of G-B over B in pts with rituximab-refractory iNHL, and demonstrates a significant improvement in OS in the G-B arm. No new safety signals were detected.
Cheson:Roche-Genentech: Consultancy; Pharmacyclics: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Astra Zeneca: Consultancy; Spectrum: Consultancy; Astellas: Consultancy; Teva: Research Funding. Trněný:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Dueck:Roche: Research Funding; Celgene: Honoraria, Research Funding; Onyx: Research Funding; Lundbeck: Honoraria, Research Funding. Lugtenburg:Celgene: Consultancy; Mundipharma: Consultancy; Servier: Consultancy; Roche: Consultancy; Takeda: Consultancy. Salles:Mundipharma: Honoraria; Gilead: Honoraria, Research Funding; Roche/Genentech: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Fingerle-Rowson:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Mattiello:F. Hoffmann-La Roche Ltd: Employment. Wassner-Fritsch:elisabeth.wassner_fritsch@roche.com: Employment. Sehn:Roche/Genentech: Consultancy, Research Funding; Celgene: Consultancy; Gilead: Consultancy; Janssen: Consultancy; Pfizer: Consultancy; Amgen: Consultancy; Seattle Genetics: Consultancy; Abbvie: Consultancy; Lundbeck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract