Abstract
Background: Southwest Oncology Group (SWOG) S0016 was a phase III randomized study that compared safety and efficacy of cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab (CHOP-R) with CHOP followed by consolidation with (131) iodine-tositumomab radioimmunotherapy (CHOP-RIT) for previously untreated follicular lymphoma (FL) patients. As reported previously, the study demonstrated excellent outcomes in both arms with 2-year progression-free survival (PFS) of 76% and 80% and 2-year overall-survival (OS) of 97% and 93% in the CHOP-R and CHOP-RIT arms, respectively. (Press. et al. JCO, 2012) We now report the long term outcome of patients who were treated on the S0016 study.
Methods: Between 2001 and 2008, 531 previously untreated advanced follicular lymphoma patients were randomized to receive either 6 cycles of CHOP every 3 weeks with 6 doses of rituximab (CHOP-R) or 6 cycles of CHOP every 3 weeks followed by RIT. Patients with advanced-stage disease (bulky stage II, III or IV) with any pathologic grade (1, 2 or 3) were eligible.
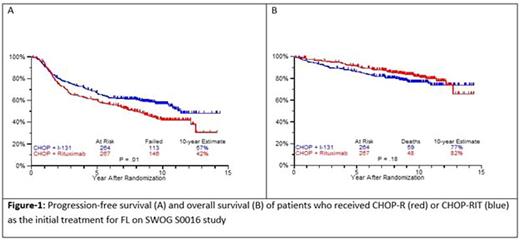
Results: Patients on CHOP-R (n=264) and CHOP-RIT (n=267) arms had balanced baseline characteristics including age (median 54.5 vs. 53.4 years), presence of B symptoms (29% vs. 26%), pathologic grade 3 (8% vs. 9%), "high" FLIPI risk group (22% vs. 26%), stage IV disease (59% vs. 63%), elevated β2M (53% vs. 55%), bone marrow involvement (56% vs. 55%) and bulky disease (24% vs. 26%). Objective remissions were documented in 226 of 267 eligible patients treated with CHOP-R (85%) and in 226 of 264 patients with CHOP-RIT (86%). Complete remission (CR) rate was 40% in the CHOP-R and 45% in the CHOP-RIT arm. Neutropenia was the most common adverse event (AE) in both arms (48% vs. 51%). Rate of grade 3-5 AEs were not different except for thrombocytopenia which was more common in the CHOP-RIT group (18% vs. 2%). After a median follow-up of 9.6 years (range 0.1 - 14.4 years), the estimated 10-year PFS was 42% (95% CI: 35.6%, 48.5%) in the CHOP-R arm and 57% (95% CI: 50.5%, 63.2%) in the CHOP-RIT arm (p-value = 0.01). 51% of responders on the CHOP-R arm and 38% of responders on the CHOP-RIT arm have relapsed (p-value= 0.01). The estimated 2-year relapse-free survival was 76% for CHOP-R patients and 81% for CHOP-RIT patients (p-value=0.15). There were 48 deaths in the CHOP+R arm and 59 deaths in the CHOP-RIT arm with an estimated 10-year OS of 82% (95% CI: 76.3%, 86.4%) with CHOP-R and 77% (95% CI: 70.5%, 81.4%) with CHOP-RIT (p-value = 0.18). (Figure-1) During the follow-up, 41 patients (15.4%) in the CHOP-R arm and 37 patients (14%) in the CHOP-RIT arm developed secondary malignancies. MDS/AML were more common in the CHOP-RIT arm (11 patients; 4.2%) compared to CHOP-R arm (5 patients; 1.9%) with a relative risk of 2.2 (95% CI: 0.8-6.3) (P-value 0.12).
Conclusion: With almost 10 years of follow-up, patients with FL treated on protocol S0016 show outstanding survival in both CHOP-R and CHOP-RIT arms, and represent the best published long-term outcomes to date in this disease. Indeed, almost half of patients enrolled still remain progression-free, confirming registry experiences. While CHOP-RIT provided significantly longer PFS and lower relapse rate compared to CHOP-R in our study, the OS rate was not different between 2 arms due in part to higher incidence of fatal MDS/AML in the CHOP-RIT group, emphasizing the importance of long-term follow-up, and calling into question the validity of PFS as a surrogate for OS in this setting. Given these outstanding outcomes, we believe chemoimmunotherapy should remain the standard induction approach for patients with high-risk FL until long-term follow-up of alternative approaches demonstrates superiority.
Support: NIH/NCI grants CA180888, CA180819, CA180821 and in part by GlaxoSmithKline
Shadman:Emergent: Research Funding; Acerta: Research Funding; Gilead: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding. Rimsza:NCI/NIH: Patents & Royalties: L.M. Rimsza is a co-inventor on a provisional patent, owned by the NCI of the NIH, using Nanostring technology for determining cell of origin in DLBCL.. Gopal:Paid Consultancy- Gilead, Janssen, Seattle Genetics, Spectrum, Research funding- Gilead, Janssen, Pfizer, BMS, Merck, Teva, Takeda, Spectrum, Seattle Genetics: Consultancy, Honoraria, Research Funding. Maloney:Juno Therapeutics: Research Funding; Genentech/Roche: Honoraria. Cheson:Gilead: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding. Smith:Amgen: Other: Educational lecture to sales force; TGTX: Consultancy; Celgene: Consultancy; Pharmacyclics: Consultancy; Genentech: Consultancy, Other: on a DSMB for two trials ; Portola: Consultancy; Juno: Consultancy; Gilead: Consultancy; AbbVie: Consultancy. Fisher:Seattle Genetics: Consultancy; Gilead: Consultancy; Johnson and Johnson: Consultancy. Friedberg:Bayer: Other: Data Safety Monitoring Committee. Press:Roche / Genentech: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal