Abstract
Background
Treatment of AML among the elderly is challenging due to intolerance of intensive therapy and greater prevalence of therapy-resistant biology. Hypomethylating agents (HMAs) are commonly used in this setting, but yield suboptimal remission rates and modest survival (Dombret 2015, Kantarjian 2012). Vadastuximab talirine (33A) is a CD33-directed antibody conjugated to 2 molecules of a pyrrolobenzodiazepine (PBD) dimer. Upon binding, 33A is internalized and transported to the lysosomes where PBD dimer is released via proteolytic cleavage of the linker, crosslinking DNA, and leading to cell death. In preclinical studies, HMA priming followed by 33A resulted in upregulated CD33 expression, increased DNA incorporation of the PBD dimer, and enhanced cytotoxicity.
Methods
A combination cohort in a phase 1 study (NCT01902329) was designed to evaluate the safety, tolerability, pharmacokinetics, and antileukemic activity of 33Ain combination with an HMA. Eligible patients (ECOG status 0-1) must have had previously untreated CD33-positive AML andhad declined intensive therapy. A single dose level of 33A, 10 mcg/kg,was administered outpatient IV every 4 weeks on the last day of HMA (azacitidine or decitabine [5-day regimen], standard dosing). Investigator assessment of response was per IWG criteria; CRi required either platelet count of ≥100,000/µL or neutrophils of ≥1,000/µL (Cheson 2003).
Results
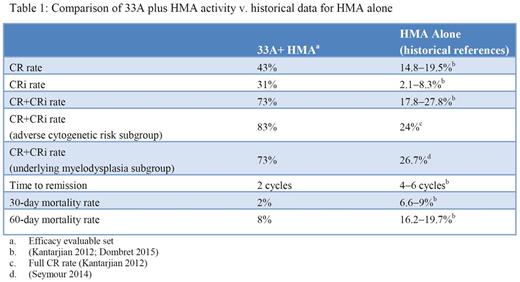
Fifty-three patients (median age 75 years; range, 60-87) have been treated with 33A+HMA. All patients had adverse (38%) or intermediate (62%) cytogenetic risk by MRC criteria; 40 patients (75%) were considered unfit for intensive therapy and 13 patients (25%) declined intensive therapy.Of the patients with secondary AML (23/53, 43%), median age was 77 years (range 60-87) with most of the patients ≥75 years (70%). The median treatment duration is currently 19.3 weeks (range, 2-86) with 13 patients remaining on treatment; no DLTs or infusion reactions were reported. G3 or higher AEs reported in ≥15% of patients were thrombocytopenia (55%), anemia (43%), febrile neutropenia (43%), neutropenia (38%), pneumonia (19%), and leukopenia (17%); no G4 or 5 bleeding events were observed. Other non-hematologic treatment-emergent AEs regardless of relationship to study treatment and reported in ˃25% of patients were fatigue (58%), nausea (47%), constipation (43%), decreased appetite, peripheral edema (40% each), pyrexia (32%), dyspnea (28%), diarrhea (26%), and dizziness (25%). 30- and 60-day mortality rates were 2% and 8% with no treatment-related deaths reported. A total of 37% (90/246) of doses were delayed due to AEs primarily related to myelosuppression (neutropenia 16%, thrombocytopenia 6%, febrile neutropenia 4%).Thirty-six of the 49 efficacy evaluable patients (73%)achieved CR (21, 43%) or CRi (15, 31%); an additional 4 patients were not efficacy evaluable by protocol definition, due to death (n=2) or withdrawal of consent (n=2) before a response assessment marrow could be obtained. Remissions were achieved after a median of 2 cycles (range, 1-4) and were observed in most of the patients with adverse risk disease including antecedent myelodysplasia (16/22, 73%), adverse cytogenetics (15/18, 83%), FLT3/ITD (5/5, 100%), and patients≥75 years (17/26, 65%). Seventeen of 22 efficacy-evaluable patients with secondary AML (77%) achieved CR (11, 50%) or CRi (6, 27%). Of all responding patients, 17 of 36 (47%) achieved MRD negativity by flow cytometry. The median relapse-free survival was 9.1 months (range, 0.1-16.5+) andOS continues to evolve with22 patients (42%) alive with a median follow-up of 10 months.
Conclusions
The combination of 33A+HMA is well tolerated with no identified pattern of off-target toxicity. Activity with the combination appears markedly improved when compared to the historical experience of HMA monotherapy in this patient population (Table 1). The CR+CRi rate of 73% in older AML patients with poor risk factors in the setting of low early mortality is particularly encouraging. Activity was maintained even in the highest risk patient groups (adverse risk cytogenetics, underlying myelodysplasia, secondary AML, FLT3/ITD). Survival data are evolving and compare favorably to historical controls. CASCADE, a phase 3 trial investigating 33A+HMA v. HMA alone in older AML patients is now enrolling (NCT02785900).
Fathi:Merck: Other: Advisory Board participation; Agios Pharmaceuticals: Other: Advisory Board participation; Bexalata: Other: Advisory Board participation; Seattle Genetics: Consultancy, Other: Advisory Board participation, Research Funding; Celgene: Consultancy, Research Funding. Erba:Gylcomimetics: Other: DSMB; Millennium Pharmaceuticals, Inc.: Research Funding; Jannsen: Consultancy, Research Funding; Ariad: Consultancy; Sunesis: Consultancy; Pfizer: Consultancy; Celator: Research Funding; Juno: Research Funding; Seattle Genetics: Consultancy, Research Funding; Agios: Research Funding; Astellas: Research Funding; Novartis: Consultancy, Speakers Bureau; Incyte: Consultancy, DSMB, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy, Research Funding; Daiichi Sankyo: Consultancy. Stein:Celgene: Other: Advisory Board, Research Funding; Agios Pharmaceuticals: Other: Advisory Board, Research Funding; Seattle Genetics: Research Funding; Novartis: Consultancy. Ravandi:Seattle Genetics: Consultancy, Honoraria, Research Funding; BMS: Research Funding. Faderl:JW Pharma: Consultancy; Amgen: Speakers Bureau; Karyopharm: Consultancy, Research Funding; Ambit Bioscience: Research Funding; BMS: Research Funding; Celator Pharmaceuticals: Research Funding; Astellas: Research Funding; Pfizer: Research Funding; Seattle Genetics: Research Funding; Celgene: Consultancy, Research Funding. Advani:Seattle Genetics: Consultancy, Research Funding. DeAngelo:Baxter: Consultancy; Pfizer: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Ariad: Consultancy; Incyte: Consultancy; Novartis: Consultancy. Kovacsovics:Seattle Genetics: Research Funding. Jillella:Seattle Genetics: Research Funding. Levy:Seattle Genetics: Research Funding; Jansen: Speakers Bureau; Amgen: Speakers Bureau; Millennium: Speakers Bureau. O'Meara:Seattle Genetics: Employment, Equity Ownership. Ho:Seattle Genetics: Employment, Equity Ownership. Stein:Seattle Genetics: Research Funding; Amgen: Consultancy, Research Funding, Speakers Bureau; Stemline Therapeutics: Consultancy, Research Funding; Argios: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.