Abstract
Introduction: The phase 3 Kids B-LONG study (NCT01440946) demonstrated the safety, efficacy, and pharmacokinetics of prophylactic recombinant factor IX Fc fusion protein (rFIXFc) for the prevention and treatment of bleeding episodes in children with severe hemophilia B. The ongoing rFIXFc extension study B-YOND (NCT01425723) is evaluating long-term safety and efficacy of rFIXFc in children and adults with hemophilia B. The safety and efficacy data for pediatric subjects from the second B-YOND interim data cut (11 Sep 2015) are reported here.
Methods: The Kids B-LONG study enrolled males aged <12 years with severe hemophilia B (≤2 IU/dL endogenous factor IX [FIX] activity) who had ≥50 exposure days (EDs) of a prior FIX product. Subjects completing Kids B-LONG (median time on study: 49.4 wk) could enroll in 1 of 3 prophylactic treatment groups in B-YOND: weekly prophylaxis (WP; 20-100 IU/kg every 7 d), individualized prophylaxis (IP; 100 IU/kg every 8-16 d), or modified prophylaxis (MP, to optimize prophylaxis with IP or WP). Subjects could change treatment groups at any point in B-YOND. The primary endpoint was development of inhibitors (neutralizing antibody value ≥0.6 BU/mL as measured by the Nijmegen-modified Bethesda assay). Secondary outcomes included annualized bleeding rate (ABR) and rFIXFc exposure days (EDs). Additional outcomes included adverse events (AEs) and evaluation of treatment of bleeding episodes. This analysis reports data for pediatric B-YOND subjects (includes subjects who were <12 y at enrollment into Kids B-LONG) treated with ≥1 dose of rFIXFc.
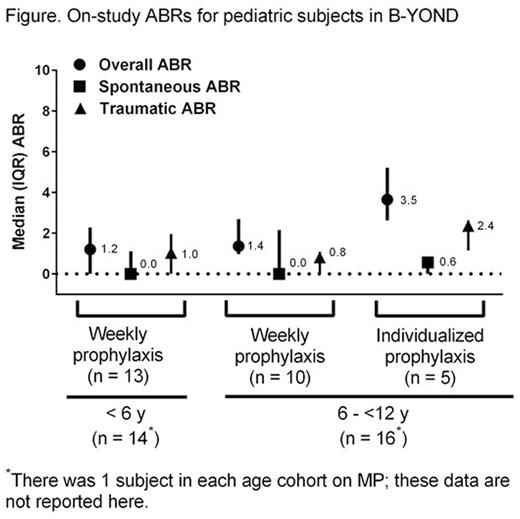
Results: At the time of the second B-YOND interim data cut, 27 subjects had completed Kids B-LONG and enrolled in B-YOND (<6 y cohort, n=13; 6 to <12 y cohort, n=14). Ten subjects had completed (ie, ended participation in the study without premature discontinuation), 16 subjects were ongoing in B-YOND, and 1 subject withdrew due to subject request. From the start of Kids B-LONG to the second B-YOND interim data cut, subjects had a median (range) 2.3 y (0.9-3.0 y) of treatment with rFIXFc, and a median (range) 127.0 (50.0-183.0) cumulative rFIXFc EDs.No inhibitors were observed. AEs were generally typical of the pediatric hemophilia B study population. There were no reports of serious allergic reactions or anaphylaxis associated with rFIXFc, and no vascular thrombotic events; no subjects had AEs related to the study drug. Median (interquartile range [IQR]) overall, spontaneous, and traumatic ABRs were low in both age groups for subjects in the IP and WP treatment groups (Figure; 1 subject in each age cohort was in the MP group [data not shown]). Median (IQR) joint ABRs were 0.0 (0.0-2.2) for subjects < 6 years in the WP treatment group and 0.9 (0.0-2.7) and 1.1 (0.0-2.4) for subjects 6 to <12 years in WP and IP treatment groups, respectively. Regardless of age or treatment group, the majority of bleeding episodes were controlled with 1-2 intravenous injections (<6 y, 97.3%; 6 to <12 y, 97.2%). At the end of Kids B-LONG, 26/27 subjects were dosing once weekly and 1/27 subject was dosing every 5 days. Compared with these dosing intervals, 14.8% of subjects lengthened, 77.8% of subjects did not change, and 7.4% of subjects decreased their prophylactic dosing interval during B-YOND. The median (IQR) dosing interval during B-YOND for pediatric subjects in the IP treatment groups was 10.0 [10.0, 10.7] days. Compared with the end of B-LONG, the median (IQR) total weekly prophylactic dose of rFIXFc was similar at the second B-YOND interim data cut (60.0 [50.0-60.0] vs 60.0 [57.0-70.0]).
Conclusion: These data confirm the long-term safety of rFIXFc with maintenance of low ABRs and extended-interval prophylaxis in children with hemophilia B.
This research was funded by Biogen and Sobi. Biogen and Sobi reviewed and provided feedback on the abstract. The authors had full editorial control of the abstract and provided their final approval of all content.
Ducore:Octapharama: Membership on an entity's Board of Directors or advisory committees; Baxalta (Shire): Membership on an entity's Board of Directors or advisory committees; LFB: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Biogen: Membership on an entity's Board of Directors or advisory committees. Fischer:Baxter: Consultancy, Research Funding, Speakers Bureau; NovoNordisk: Consultancy, Research Funding, Speakers Bureau; Octapharma: Speakers Bureau; Biogen: Consultancy; Biotest: Consultancy, Speakers Bureau; Baxalta/Baxter: Consultancy, Research Funding, Speakers Bureau; Wyeth: Research Funding; Pfizer: Consultancy, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Speakers Bureau; Bayer: Consultancy, Research Funding, Speakers Bureau; Freeline: Consultancy. Kulkarni:Kedrion: Membership on an entity's Board of Directors or advisory committees; BPL: Membership on an entity's Board of Directors or advisory committees; Biogen: Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxter: Membership on an entity's Board of Directors or advisory committees, Research Funding. Nolan:Sobi: Research Funding; Biogen: Research Funding. Perry:Biogen: Consultancy, Honoraria; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees. Yuan:Biogen: Employment, Equity Ownership. Ramirez-Santiago:Biogen: Employment, Equity Ownership. Ferrante:Sobi: Employment. Lethagen:Sobi: Employment.
Author notes
Asterisk with author names denotes non-ASH members.