Abstract
Background: Thecross talk between neoplastic cells and microenvironment is mediated by direct cell-to-cell contacts, secretion of soluble factors and release of extracellular vesicles (EVs). EVs deriving from tumor cells in chronic lymphocytic leukemia (CLL) may affect the surrounding microenvironment, playing a key role on survival of neoplastic clone. Of note, EVs are increased in CLL patients compared to normal subjects. In this study, we performed a comprehensive characterization of serum EVs from previously untreated CLL patients, investigating, in particular, phenotype and absolute number, in order to test their possible prognostic significance.
Patients and methods: Serum samples of 131 newly diagnosed CLL and from 28 healthy subjects were analyzed. One milliliter of serum was processed with serial ultracentrifugations. Each sample of EV-enriched pellet, from patients and controls, was freshly analyzed by FACS Calibur (Becton Dickinson BD) cytometer using Cell Quest software (BD). The system was calibrated using standard microbeads with a diameter of 0.3-0.9-3 μm to define the size limit for microvesicles (MV), a subtype of EVs. To determine the number of MV/μL serum, TruCOUNT beads (BD) were added immediately prior to analysis by flow cytometry. MV morphology was characterized by transmission electron microscope (TEM). MV were then labeled with fluorochrome-conjugated monoclonal antibodies (anti-CD19, CD3, CD94, CD20, CD2, CD56, CD52, CD37) and their specific isotypic controls. Finally, MV were correlated with the main clinical and biological disease's characteristics, including clinical outcome.
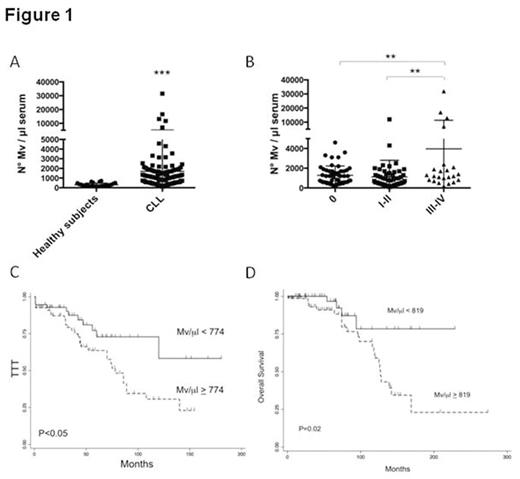
Results: Flow cytometric analysis of MVs showed a size within 1mm, based on forward and side scatter evaluation and the use of standard beads. The analysis was carried out on MV population isolated by the gating strategy. MV were also visualized by TEM, showing a spheroid morphology. We found a significantly higher mean number of MV in CLL patients with respect to healthy subjects (p<0.001) (Fig.1A). Moreover, by stratifying CLL patients for Rai stage, those with advanced clinical stages (III-IV) had a significantly higher number of MV with respect to patients with Rai stages 0 and I-II (p<0.01) (Fig.1B). To confirm the neoplastic cell source, MV were analyzed for the expression of lineage-specific antigens. In particular, B cells released preferentially CD19+ and CD37+ MV as compared to CD20. As observed for the total number of MV, significant increased amounts of CD19+, CD20+ and CD37+ MV were found in advanced clinical stages. Absolute MV number cut-off selected by ROC analysis distinguished Rai stage 0 patients with shorter time to treatment (TTT) from those with more stable disease (median 75 months vs not reached, P<0.01). Likewise, in the entire cohort, two groups of patients with different TTT (median 78 months vs not reached, P<0.01) (Fig.1C) and different overall survival (OS) (median 127 months vs not reached, P=0.02) (Fig.1D) were identified. At multivariate analysis, serum MV independently predicted for TTT (along with Rai stage, lymphocyte count and CD38 expression) and OS (along with Rai stage).
Conclusions: Our study indicates that: (i) MV number is higher in CLL patients as compared to normal controls; (ii) CD19 and CD37 are the most represented B-cell antigens on CLL derived MV; (iii) total MV levels are associated with high tumor burden; (iv) total MV levels predict for TTT in Rai 0 patients, as well as for TTT and OS in all stage patients. These observations suggest that MV may represent a new biomarker for CLL.
D'Arena:Janssen-Cilag: Honoraria. Musto:Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.