Abstract
Background: CPI-613 is a first in class agent that inhibits pyruvate dehydrogenase (PDH) and a-ketogluterate dehydrogenase. We have previously shown CPI-613 inhibits mitochondrial respiration, causes phosphorylation of PDH, activation of adenosine monophosphate activated kinase (AMPK) in AML cells and was well tolerated by patients with myeloid malignancies.
Methods: We completed a phase I trial of CPI-613 in combination with high dose cytarabine (HiDAC) and mitoxantrone for relapsed or refractory AML patients. RNA sequencing analysis was conducted on a subset of patient samples to explore gene expression profiles associated with response. To explore genetic determinants of response, Cas-9 expressing AML cells were used to knock out p53 and AMPK.
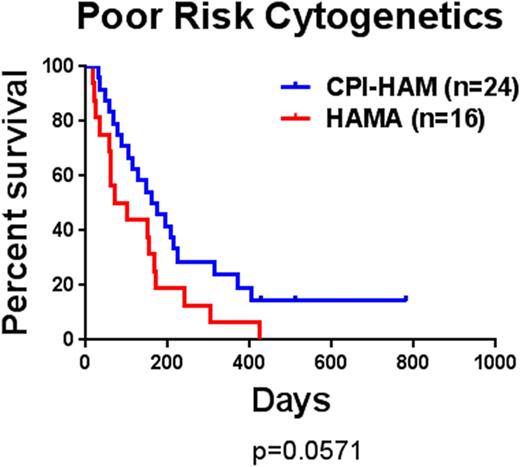
Results: In the phase I trial 67 patients were enrolled and 62 were evaluable. One patient died before completion of therapy, three died prior to the day 14 nadir marrow and one patient with accelerated phase CML was enrolled in error. The median age was 60 years (range 21-79). In patients with relapsed disease the median duration of CR1 was 11.2 months. Cytogenetics were poor risk in 27 patients, intermediate in 33, and good in 6. In the 62 evaluable patients the results were as follows. The overall response rate was 50% (26CR+5CRi) with a median survival of 6.7 months. Response was significantly associated with survival with a median survival of 13.2 months in responders compared to 3 months for all others. In patients ≥60 years old the CR/CRi rate was 47% (15/32) with a median survival of 6.9 months. This was not significantly different from patients younger than 60 years old. Surprisingly, the response rate for patients with poor risk cytogenetics was 46% (9CR+2CRi/24) with a median survival of 5.5 months. In a historical cohort, only 19% (3/16) of patients with poor risk cytogenetics responded with a median survival of 2.8 months (Figure 1). The most common toxicities of CPI-613 were diarrhea and nausea. Thirteen patients (21%) went on to allogeneic stem cell transplantation. Several patients had blood samples taken before and after CPI-613 infusion. Three of these patients had increased phosphorylation of PDH consistent with its inhibition. Additionally, two patients demonstrated a robust phosphorylation of AMPK consistent with preclinical studies. The role of AMPK in response was assessed by competition assays using Cas-9 expressing AML cells that have deleted AMPK. Normal cells out-competed AMPK deleted cells when treated with CPI-613 but not when treated with cytarabine, suggesting AMPK is a specific resistance factor against TCA cycle inhibition. To explore if loss of p53 function (common in poor risk cytogenetics) affected response we tested CPI-613 alone or with an anthracycline against an AML cell line with and without p53 knockdown. Suppression of p53 function resulted in significant resistance to anthracyclines but no change in response to CPI-613; the combination enhanced cell kill over either agent alone. To gain insight into the transcriptional programs that distinguish responders from non-responders prior to treatment, we sequenced the transcriptomes of mononuclear cells from 25 baseline patient samples: 19 from bone marrow and 9 from peripheral blood. These samples corresponded to 14 responders and 11 non-responders. Analysis for enrichment of gene ontologies revealed several significant biological categories for the genes overexpressed in responders, while none were identified for the non-responders. The responders showed significant enrichment for gene categories related to immune involvement, particularly genes associated with B lymphocytes.
Conclusions: TCA cycle inhibition with CPI-613 is a promising novel approach in the treatment of AML patients, especially in the elderly and those with poor risk cytogenetics. Low levels of AMPK may identify patients with an increased likelihood of response. Finally, the presence of gene expression profiles consistent with immune cells raises the possibility that in addition to direct effects on leukemia cells, TCA cycle inhibition may alter microenvironment immune responses.
Kaplan-Meier curves of poor risk cytogenetic patients treated with CPI-613, HiDAC and mitoxantrone (CPI-HAM) compared to a historical cohort of patients treated with HiDAC, mitoxantrone and asparaginase (HAMA).
Kaplan-Meier curves of poor risk cytogenetic patients treated with CPI-613, HiDAC and mitoxantrone (CPI-HAM) compared to a historical cohort of patients treated with HiDAC, mitoxantrone and asparaginase (HAMA).
Pardee:Cornerstone Pharmaceutical: Consultancy; Celgene: Speakers Bureau; Novartis: Speakers Bureau; Merck: Consultancy. Manuel:Novartis: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.