Abstract
Introduction
Solid tumors are infiltrated by a variety of myeloid-derived cells (MDCs), such as tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs). Extensive studies have revealed that they play pivotal roles in tumor progression, such as immunosuppression, angiogenesis, and enhancing tumor cell invasion, and metastasis. It has been suggested, however, that undefined MDCs exist in tumors and play key roles in tumor progression. In order to develop a novel anti-tumor strategy, we have been searching undefined tumor-infiltrating MDCs that associate with tumor progression. Recently we have isolated adherent MDCs (AMCs), which strongly adhere to culture dishes, from subcutaneous tumors of Lewis lung carcinoma (LLC). AMCs contained CD45(+) CD11b(+) F4/80(-) undefined cell population (named F4/80(-)AMC) that exhibit protumoral functions. In this research, we characterized F4/80(-)AMC and explored its protumoral functions.
Methods
F4/80(-)AMC were subcutaneously transplanted into syngenic C57BL/6J mice with firefly luciferase-expressing LLC (LLC/luc) cells and bioluminescence (BL) signals corresponding to tumor burden were monitored every four days. The direct effect of F4/80(-)AMC on cancer cell growth was examined in vitro by culturing LLC/luc cells with (co-culture) or without (mono-culture) F4/80(-)AMC. In addition, conditioned medium (CM) collected from co-culture and mono-culture were analyzed by using mouse cytokine protein array. Receptors for markedly increased cytokines in co-culture CM were knocked down in LLC/luc cells to examine their involvement in LLC growth, and the candidate cytokines were further investigated for their direct effect on LLC growth by adding their recombinant proteins to the culture medium and monitoring the growth of LLC/luc cells. Furthermore, the possibility that F4/80(-)AMC could have a role to recruit other MDCs to tumor site was examined by in vitro chemotaxis assays using transwell chambers, and the impact of the neutralizing antibody against candidate cytokines on MDCs chemotaxis was evaluated.
Results
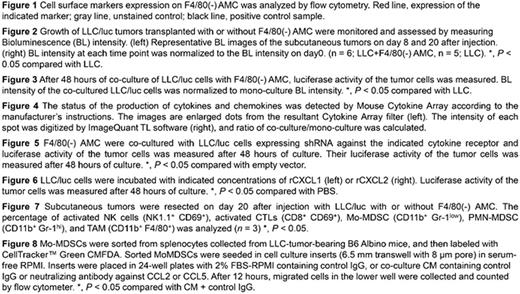
Investigation of surface makers on F4/80(-)AMC revealed that F4/80(-)AMC is distinct from any other already-known myeloid cells [Figure 1]. BL signal from tumors co-transplanted with F4/80(-)AMC was significantly increased compared to the signal from LLC/luc tumors, indicating that F4/80(-)AMC promotes tumor growth in subcutaneous tumor models [Figure 2]. Since F4/80(-) AMC was able to enhance proliferation of LLC cells in an in vitro co-culture without cell-cell contact [Figure 3], we hypothesized that F4/80(-)AMC may directly enhance tumor cell growth through cytokine release. Cytokine array showed that 6 cytokines (lipocalin-2, CXCL1, CXCL2, adiponectin, CCL2, and CCL5) were markedly increased in co-cultured CM compared to mono-cultured CM [Figure 4]. Knocking down (KD) of receptors for these cytokines indicate that only KD of CXCR2, a receptor for CXCL1/CXCL2, significantly abrogated F4/80(-)AMC-induced LLC growth [Figure 5]. CXCL1 and CXCL2 dose-dependently promoted LLC proliferation [Figure 6], demonstrating that F4/80(-)AMC directly enhance cancer cell proliferation via CXCL1 and CXCL2. Furthermore, in tumors co-transplanted with F4/80(-) AMC, monocytic MDSC (Mo-MDSC) and TAM were elevated, while activated CTLs were reduced [Figure 7]. It has been known that Mo-MDSC can suppress CTLs activities and that TAM is differentiated from Mo-MDSC in tumors. Therefore, above results may reflect F4/80(-)AMC-mediated recruitment of Mo-MDSC, which subsequently suppress CTLs and differentiate into TAM. Moreover, antibodies against CCL2 and CCL5 significantly suppressed the migration of Mo-MDSC toward CM of F4/80(-) AMC, suggesting that F4/80(-)AMC recruits Mo-MDSC via CCL2 and CCL5 secretion [Figure 8]. Taken together, these results strongly suggest that F4/80(-)AMC contributes to tumor progression by creating an immunosuppressive microenvironment.
Conclusions
Our study sheds light on the protumoral functions of novel MDCs: F4/80(−)AMC. Further characterization of F4/80(−)AMC and elucidation of its relationship with known MDCs are required to understand overall roles of MDCs in malignant progression. Our goal in this work is to identify the cell surface markers of F4/80(−)AMC and develop a novel treatment strategy for advanced cancers based on the knowledge of F4/80(−)AMC.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.