Abstract
Introduction:
Allogeneic hematopoietic stem cell transplantation (HCT) is a treatment for CLL that can give long disease control. Even with the availability of kinase and BCL2 inhibitors, HCT is still performed in fit patients (pts) with high-risk CLL. Almost exclusively, outcomes on matched related and unrelated donor transplantations in CLL have been published. Recently, mismatched related donors are gaining interest because of the better outcome of haploidentical HCT with post-transplantation cyclophosphamide (PTCY).
Methods:
All pts with CLL who received a first allogeneic HCT with a mismatched related donor and whose data were available in the EBMT registry were analyzed. Median values and ranges are reported for continuous variables and percentages for categorical variables. The probabilities of overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method and the log-rank test for univariate comparisons. Relapse/progression and nonrelapse mortality (NRM) were analyzed together in a competing risk framework. Statistical analyses were performed using SPSS and R.
Results:
One-hundred-seventeen pts with CLL (74% males) underwent a mismatched related donor transplantation between 1984 and 2015 (1984-1999: 10, 2000-2004: 18, 2005-2009: 23, 2010-2016: 66). Median follow-up after HCT was 8 months (range 0-187 months). Median age at transplantation was 54 years (yrs) (range 27-71 yrs). Median time from diagnosis to HCT was 67 months (range 4-207 months). Eighteen pts (17%) had previously undergone autologous stem cell transplantation (ASCT). Disease status at HCT was CR in 16% of pts, PR in 39% and SD/PD in 45%. The Karnofsky score was known for 98 pts; 96% had a score of 70% or more at the time of HCT. Fifty-eight percent of pts received reduced-intensity conditioning, 42% myeloablative conditioning. Peripheral blood stem cells were used in 68% of pts, bone marrow in 32%. The HCT was sex matched in 41% of recipient-donor pairs. The relationship of the donor to the patient was known for 34 pts; in 53% the donor was a child, in 38% a sibling and in 6% a parent. Forty pts (38%) received PTCY as GVHD prophylaxis. In the other 77 pts various methods of T-cell depletion (TCD) were used, but not all methods were specified. At least 56% of those pts had in vivo TCD.
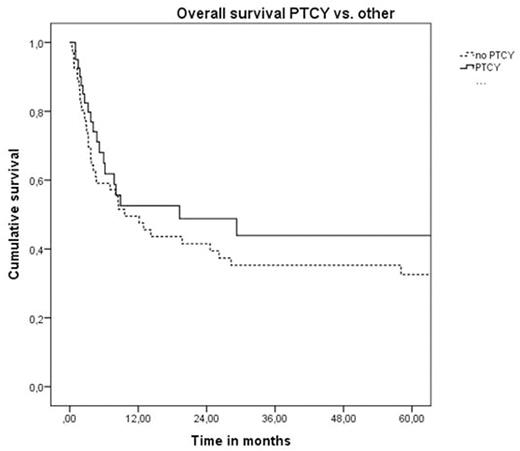
For the whole cohort of pts OS at 2 and 5 yrs was 46% and 37%, respectively. PFS at 2 and 5 yrs was 38% and 30%, respectively. The use of PTCY did not have a significant impact on OS (49% vs. 42% at 2 yrs, 44% vs. 33% at 5 yrs, p=0.35) and PFS (45% vs. 31% at 2 yrs, 40% vs. 22% at 5 yrs, p=0.15). CI of NRM in the whole group at 2 and 5 yrs were 41% and 45%, respectively. CI of relapse at 2 and 5 yrs were 21% and 25%, respectively. The CI of NRM and relapse at 2 and 5 yrs were not statistically different in pts who received PTCY compared to other types of TCD (NRM: 38% vs. 45% at 2 yrs, 43% vs. 49% at 5 yrs, p=0.45; relapse: 17% vs. 25% at 2 yrs, 17% vs. 29% at 5 yrs, p=0.33). For the whole cohort, the incidence of acute graft-versus-host disease (aGVHD) at 100 days was 34% for grade II-IV and 16% for grade III-IV with a median time of onset of 23 days (range 4-57 days).
Conclusions:
Mismatched related donor HCT resulted in a 5-year PFS in 30% of the pts. This result seems only slightly inferior to matched donor transplant (5 yrs PFS 37%1). NRM was higher than expected in this cohort, but comparable to other studies on haploSCT with in vivo T-cell depleted grafts. In conclusion, a mismatched related donor HCT may be considered for high-risk chemoimmunotherapy-refractory or 17p deleted/TP53 mutated CLL pts without options for kinase and BCL2 inhibitor therapy. More data are needed to assess the value of PTCY for GVHD prophylaxis in this specific context.
References:
1. Schetelig J, de Wreede L, Moreno C, et al. Risk factors for adverse outcome in patients with Chronic Lymphocytic Leukemia (CLL) undergoing Allogeneic Hematopoietic Cell transplantation (alloSCT): a Retrospective EBMT Analysis. Abstract WP024, EBMT meeting 2015.
Ciceri:MolMed SpA: Consultancy. Foà:Ariad: Speakers Bureau; Pfizer: Speakers Bureau; BMS: Consultancy; Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Janssen-Cilag: Consultancy, Speakers Bureau; Genetech: Consultancy; Roche: Consultancy, Speakers Bureau. Hallek:Mundipharma: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; F. Hoffmann-LaRoche: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau. Schetelig:Sanofi: Honoraria. Kröger:Sanofi: Honoraria, Research Funding; Neovii: Honoraria, Research Funding; Riemser: Honoraria, Research Funding; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.