Abstract
Introduction
Tumors deregulate immunological antitumor response, resulting in survival of tumor cells, which implicate the existence of immunological tolerance to tumors.CD4+ T cells activate tumor-specific cytotoxic CD8+ T cells via cytokines and they also can eliminate cancer in the absence of CD8+ T cell. Absolute CD4+ T-cell count(ACD4C)in biopsied specimen is known to correlate with therapeutic outcomes in DLBCL. In patients with solid cancer, CD4+ T cells decrease in the peripheral blood, whereas regulatory T cells (Tregs) increase in the peripheral blood.Tregshave a role to reduce antibody dependent cellular toxicity (ADCC) of rituximab against CD20+ B-cell malignancies. On the other hand,we and othersknow that absolute lymphocyte count in peripheral blood can predict survival of diffuse large B-cell lymphoma (DLBCL). It has been indefinite, however, which lymphocyte including CD4+ T cells in peripheral blood reflect the prognosis of DLBCL.
Method
We enrolled patients who were diagnosed with de novo DLBCL from 2006 until 2013, received R-CHOP, and followed up at Cancer Institute Hospital, Tokyo, Japan. We had measured absolute lymphocyte count, T-cell ratio, CD4+ T-cell ratio, and CD8+ T-cell ratio in these patients using pretreatment blood samples. Data were collected prospectively and recorded into a computerized database. All patientsgave written informed consent allowing the use of their medical record. The optimal cut-off values were made based on its utility as a marker for death using box plot, clinical important value from references, and receiver operating characteristic curve. Differences between the results of comparative tests were considered significant if the two-sided P value was less than 0.05.
Results
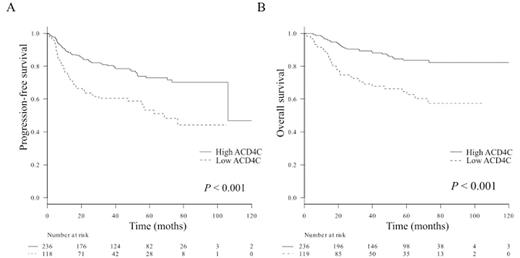
A total of 355 patients were diagnosed with de novo DLBCL.Baseline characteristics were following: median age was 65 (range 20-89), Patients aged over 60 were 243 (68%), male to female ratio was 1.2, ECOG PS ≥ 2 of 19 (5%), elevated LDH of 152 (43%), low ACD4C (< 350 x 106 /l) of 119 (34%), low ACD8C (< 300 x 106 /l) of 144 (41%), CD5+ DLBCL of 38 (11%), Ann Arbor stage III/IV of 145 (41%), and involved extranodal sites ≥ 2 of 93 (26%). Germinal center B cell (GCB) DLBCL was seen in 167 (53%), non-GCB DLBCL was seen in 148 (47%). Patients without evidence of death (n = 282) at last follow-up had a higher ACD4C (≥ 350 x 106 /l) at diagnosis than those with death (n = 73) (P < 0.0001). There was also markedly difference in absolute CD8+ T-cell count, but no difference in absolute B-cell count. At the median follow-up of 57 months, Kaplan-Meier method estimated that 5-year PFS was 78.1% in the high ACD4C group and 62.0% in the low ACD4C group (Figure 1A, log-rank P < 0.001), whereas 67.4% in the high ACD8C group and 41.6% in the low ACD8C group (P = 0.01). Furthermore, 5-year OS was 83.6% in the high ACD4C group and 64.5% in the low ACD4C group (Figure 1B, log-rank P < 0.001), whereas 56.2% in the high ACD8C group and 36.1% in the low ACD8C group (P < 0.01). An ACD4C < 350 x 106 /l was identified as an adverse prognostic marker in DLBCL by Cox hazard model (hazard ratio 1.9, P = 0.01). In addition, CD5+ DLBCL, PS ≥ 2, stage III/IV, and non-GCB DLBCL were identified as low ACD4C. Age > 60, extranodal diseases ≥ 2, and elevated LDH were not identified in this study. ACD4C had negative correlation with tumor burden, which was shown by Pearsonfs coefficient (correlation with LDH; r = -0.24, P < 0.0001) and Studentfs t-test (correlation with stage; P < 0.0001). Interestingly, low ACD4C affected OS only in the stage III/IV, non-GCB DLBCL, and high-IPI groups (fisherfs exact test P < 0.01). Baseline characteristics of the low ACD4C group showed higher rate of stage III/IV (P < 0.001), elevated LDH (P < 0.01), extranodal disease ≥ 2 (P < 0.001), soluble IL-2 receptor > 2000 U/l (P < 0.001), low serum albumin (P = 0.001), and beta2 microglobulin > 2 mg/dl (P < 0.001).
Conclusion
This study demonstrates that ACD4C had a negative correlation with tumor burden and low ACD4C at diagnosis made worse prognosis of patients with DLBCL, in particular, those who had high tumor burden, non-GCB, or high IPI at diagnosis, suggestingTregsmight increase in peripheral blood in the low ACD4C group and might impair the ADCC of rituximab.
Terui:Yanssen: Honoraria. Mishima:Chugai: Consultancy. Nishimura:Chugai: Consultancy. Yokoyama:Chugai: Consultancy. Hatake:Meiji-Seika: Consultancy; Kyowa Kirin: Honoraria, Research Funding; Chugai: Research Funding; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.