Abstract
Heparin-induced thrombocytopenia (HIT) is characterized by antibodies (abs) directed to complexes of PF4 and heparin that are an important cause of morbidity and mortality in heparin-treated patients. Direct Thrombin inhibitors (DTIs) and fondaparinux (off-label) are the mainstays of therapy, but despite their use, thrombosis is common. Thus there is a need for additional treatment regimens, especially in severely affected patients. We recently encountered two patients with severe thrombotic HIT and persistent thrombocytopenia who responded dramatically to intravenous immunoglobulin G (IVIg) and performed studies to examine the basis for this effect.
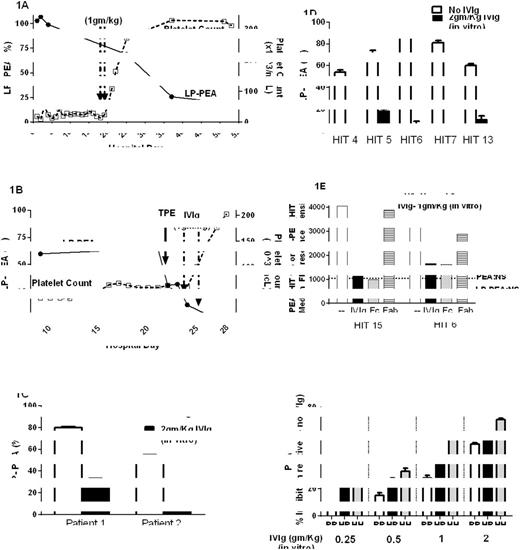
Patient 1, a 47-year-old man, presented with severe thrombocytopenia (TP, 13,000/ul), lower extremity thromboses and pulmonary embolism 13 days after being treated with heparin during cardiac surgery. PF4 ELISA (2.7 OD) and serotonin release assay (SRA) (100%) were strongly positive. Argatroban and then bivalirudin were given, but TP persisted for 17 days (Fig 1A). On Days 18 and 19 1gm/Kg of IVIg was administered. Platelet counts rose dramatically and he was discharged four days later (Fig 1A). Surprisingly, despite platelet recovery, two "platelet-activating" HIT tests, the SRA and newly described PEA (Padmanabhan et al, Chest 2016, epub) remained strongly positive (data not shown). The standard PEA is performed using platelets "primed" by pre-incubation with 30 ug/ml platelet factor 4 (PF4) and no heparin. However strong platelet-activating HIT abs, like those seen in this patient with "delayed HIT" may require only a small amount of PF4 to prime platelets for activation. When testing was repeated using platelets primed with only 1 ug/ml PF4 (Low PF4 PEA, LP-PEA), an inverse correlation between the PEA signal and platelet counts was observed (Fig 1A).
Patient 2, a 73-year-old man, presented with severe TP (25,000/ul) and pulmonary embolism 9 days after receiving heparin during cardiac surgery. PF4 ELISA (2.3 OD) and SRA (100%) were strongly positive. He was treated with bivalirudin but remained thrombocytopenic with positive HIT test results (not shown) for 3 weeks (Fig 1B). Therapeutic plasma exchange (TPE) was performed on Day 22 with no effect on platelet counts. On Days 23 and 24, 1gm/Kg of IVIg was administered. Platelet counts rose sharply and he was discharged one week later. As with Patient 1, the LP-PEA but not the PEA test results became negative after IVIg treatment, coinciding with improvement in platelet counts (Fig 1B).
To understand the beneficial effect of IVIg in these patients, we added IVIg to patient serum in amounts sufficient to produce IgG levels comparable to those achieved in vivo and examined the effects in the PEA/LP-PEA assay. At the 2gm/Kg dose, IVIg reduced the LP-PEA signal significantly in both patients (Fig 1C). Similar results were obtained with sera from 5 other patients with HIT, indicating that the IVIg effect is not restricted to HIT sera with unique characteristics (Fig 1D). Purified IgG Fc fragments are both necessary and sufficient to achieve this effect, while Fab fragments demonstrated no inhibitory activity (Fig 1E).
IgG2 comprises approximately 25% of IVIg and it is known that IgG2 binds poorly to the RR131 isotype of the platelet Fc receptor, FcGammaRIIa. Thus, RR131 platelets should be less susceptible to IVIg-based inhibition than HH131 platelets. Consistent with this, activation was more effectively inhibited with HH131 platelets than with RR131 (Fig 1F). These results were confirmed using a second HIT ab (data not shown).
Experience with these patients shows that IVIg can be highly effective for treating patients with HIT unresponsive to standard therapies. The constant domain (Fc) of IgG plays a critical role in mediating this effect. Patients homozygous for the RR131 FcGammaRIIa polymorphism may be less responsive to IVIg treatment and may require higher doses or more prolonged therapy. IVIg has been used only rarely to treat HIT. Additional studies are needed to define its role in treating this dangerous condition.
LP-PEA results shown are the means of duplicate determinations. Fig 1C, D and F. Results shown are means and SDs of triplicate determinations. Fig 1E. Results shown are representative of duplicate determinations. Fig 1F. IVIg doses at 2, 1, 0.5 and 0.25gm/Kg correspond to IVIg at a final concentration of 46, 23, 12 and 6mg/ml in the HIT sample, respectively.
LP-PEA results shown are the means of duplicate determinations. Fig 1C, D and F. Results shown are means and SDs of triplicate determinations. Fig 1E. Results shown are representative of duplicate determinations. Fig 1F. IVIg doses at 2, 1, 0.5 and 0.25gm/Kg correspond to IVIg at a final concentration of 46, 23, 12 and 6mg/ml in the HIT sample, respectively.
Padmanabhan:Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591; Fenwal (Fresenius Kabi): Research Funding; Schlesinger & Associates: Consultancy, Honoraria; LEK Consulting: Consultancy, Honoraria; Mallinckrodt Pharmaceuticals: Consultancy, Honoraria; Terumo BCT: Consultancy, Honoraria. Jones:Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591. Bougie:Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591. Aster:Bloodcenter of Wisconsin: Patents & Royalties: A patent application has been filed on a Method of detecting platelet activating antibodies that cause heparin-induced thrombocytopenia/thrombosis; PCT/US14/62591.
Author notes
Asterisk with author names denotes non-ASH members.