Abstract
Essential thrombocythemia (ET) is an indolent myeloproliferative neoplasm that may be complicated by vascular events, including both thrombosis and bleeding. This disorder may also transform into more aggressive myeloid neoplasms, in particular into myelofibrosis. The identification of somatic mutations of JAK2, CALR, or MPL, found in about 90% of patients, has considerably improved the diagnostic approach to this disorder. Genomic profiling also holds the potential to improve prognostication and, more generally, clinical decision-making because the different driver mutations are associated with distinct clinical features. Prevention of vascular events has been so far the main objective of therapy, and continues to be extremely important in the management of patients with ET. Low-dose aspirin and cytoreductive drugs can be administered to this purpose, with cytoreductive treatment being primarily given to patients at high risk of vascular complications. Currently used cytoreductive drugs include hydroxyurea, mainly used in older patients, and interferon α, primarily given to younger patients. There is a need for disease-modifying drugs that can eradicate clonal hematopoiesis and/or prevent progression to more aggressive myeloid neoplasms, especially in younger patients. In this article, we use a case-based discussion format to illustrate our approach to diagnosis and treatment of ET.
Medscape Continuing Medical Education online
This activity has been planned and implemented through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2477.
Disclosures
Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Editor Bob Löwenberg and the authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Based on the review provided in this paper, distinguish the diagnosis of essential thrombocythemia (ET).
Determine treatment of ET.
Evaluate the determination of individual patient risk as a guide to selecting therapy for ET.
Release date: November 17, 2016; Expiration date: November 17, 2017
Introduction
Essential thrombocythemia (ET) is one of the Philadelphia-negative classical myeloproliferative neoplasms (MPNs), a category of the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues that also includes polycythemia vera (PV) and primary myelofibrosis (PMF).1,2 In the last few years, there have been significant advances in our understanding of the genetic basis, pathophysiology, and clinical course of ET.3-20 Our article aims to offer up-to-date information and guidance regarding diagnosis and treatment of ET patients. To provide directions in the therapeutic management of common or complex clinical situations of the disease, we will first use clinical vignettes illustrating representative cases of ET (Table 1) and will later consider specific situations of interest.
How we diagnose ET
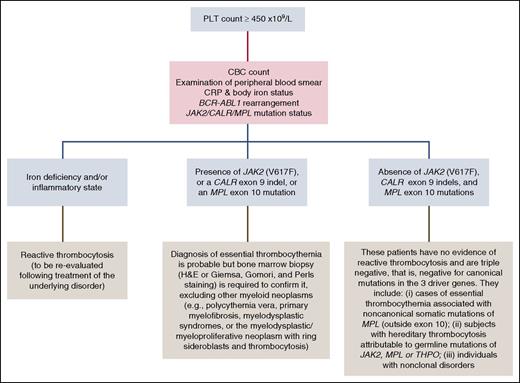
Thrombocytosis is defined as a platelet (PLT) count ≥450 × 109/L. The major types of thrombocytosis include reactive (or secondary) thrombocytosis, clonal myeloid neoplasms, and familial or hereditary thrombocytosis.21 As the most common cause is a reactive process, at the time of the first visit we always consider secondary thrombocytosis through a complete interview, physical examination, and first-level tests as reported in Table 2 and Figure 1.
Our approach to the differential diagnosis of thrombocytosis. For the analysis of JAK2/CALR/MPL mutations status, we use granulocyte DNA and perform the following tests sequentially: (1) a quantitative polymerase chain reaction–based allelic discrimination assay for JAK2 (V617F) with a sensitivity of <0.1%; (2) if JAK2 (V617F) is absent, Sanger sequencing for detection of CALR exon 9 indels; (3) if JAK2 (V617F) is absent and CALR exon 9 is wild type, a high-resolution melt assay for detection of MPL exon 10 mutations followed by Sanger sequencing in case of mutant pattern. H&E, hematoxylin and eosin.
Our approach to the differential diagnosis of thrombocytosis. For the analysis of JAK2/CALR/MPL mutations status, we use granulocyte DNA and perform the following tests sequentially: (1) a quantitative polymerase chain reaction–based allelic discrimination assay for JAK2 (V617F) with a sensitivity of <0.1%; (2) if JAK2 (V617F) is absent, Sanger sequencing for detection of CALR exon 9 indels; (3) if JAK2 (V617F) is absent and CALR exon 9 is wild type, a high-resolution melt assay for detection of MPL exon 10 mutations followed by Sanger sequencing in case of mutant pattern. H&E, hematoxylin and eosin.
Assessment of symptom burden in MPNs has shown that although ET has the lowest symptom severity, the prevalence of constitutional symptoms reported by patients is relatively high.22 It should be noted, however, that in this study patients were an average of 6.7 years out from diagnosis at survey completion. In our experience, most patients with ET are asymptomatic at diagnosis, and detection of thrombocytosis is typically incidental.
First-level laboratory tests include screening for mutations in the 3 MPN driver genes (Figure 1). In a recent study of 745 patients,10 we found that 466 (62%) carried JAK2 (V617F), 176 (24%) had CALR exon 9 indels, and 28 (4%) had MPL exon 10 mutations: only 75 (10%) patients did not carry any of the above driver mutations. In our practice, search for JAK2 (V617F) on granulocyte DNA is the initial investigation performed in all patients with suspected ET; if JAK2 (V617F) is absent, we screen for CALR exon 9 indels, and then, if this latter screening is also negative, we search for MPL exon 10 mutations.
Once the results of first-level tests are available, we follow the flowchart reported in Figure 1. The detection of a driver mutation confirms the presence of a myeloid neoplasm, but the absence does not rule out this possibility because as many as 1 in 10 ET patients can be triple negative, that is, negative for canonical mutations in the above driver genes. Two recent studies have indeed shown that a few triple-negative patients carry activating mutations of MPL outside exon 10, and that these noncanonical mutations may be either inherited or somat-ically acquired.23,24 One study has also shown that some patients carry noncanonical, activating mutations of JAK2, which appear to be germ line in most instances.23 Finally, some patients have evidence of polyclonal hematopoiesis, and most likely do not have a true MPN.23,24 Thus, the so-called triple-negative cases may include patients with ET associated with noncanonical mutations of MPL, individuals with hereditary thrombocytosis (see “Distinguishing familial ET from hereditary thrombocytosis”), and also subjects with nonclonal thrombocytosis (Figure 1).
The myelodysplastic (MDS)/MPN with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T) can mimic ET.25 Most patients with MDS/MPN-RS-T have a combination of SF3B1 mutation (as a driver genetic lesion) and JAK2, MPL, or CALR mutation (as a subclonal genetic lesion); however, up to one-third of patients may have wild-type SF3B1.25-27 Recent reports have described an atypical MPN associated with both BCR-ABL1 rearrangement and CALR mutation28-30 : this further underlines the importance of strictly adhering to the WHO criteria, screening for BCR-ABL1 in the diagnostic approach.
An interesting study has shown that about one-fourth of ET patients carry additional somatic mutations in non-MPM driver genes.14 Although in the whole population of MPN patients the presence of 2 or more somatic mutations represented a negative prognostic factor, its significance in ET patients was less clear.
In our clinical practice, we are currently using the 2016 WHO criteria for the diagnosis of ET, as reported in Table 3.2 Because these criteria have underlined the importance of distinguishing true ET from early prefibrotic stages of PMF, Table 3 also includes the 2016 WHO diagnostic criteria for prefibrotic myelofibrosis.
Clinical vignettes: establishing diagnosis
Clinical vignettes are explored in detail in Table 1.
Case 1: Low-risk JAK2-mutant ET
This woman had normal body iron status and no evidence of inflammation. JAK2 (V617F) was detected on granulocyte DNA with a low percentage of mutant alleles, which is a common finding in ET,31 and a bone marrow biopsy unequivocally confirmed the diagnosis of ET.
Case 2: Low-risk CALR-mutant ET
This young woman had normal body iron status and a normal C-reactive protein (CRP). Because JAK2 (V617F) was not detected on granulocyte DNA, CALR exon 9 was sequenced, and the type 1 CALR mutation (c.1092_1143del, L367fs*46) was detected. Bone marrow biopsy showed normal age-adjusted cellularity, and an increased number of giant megakaryocytes; reticulin fibrosis was absent. A final diagnosis of CALR-mutant ET was performed; CALR mutation is often associated with a very high platelet count,10,11 as in this case.
Case 3: JAK2-mutant ET progressing to PV
The initial diagnosis of ET was based on the complete blood cell (CBC) count, positivity for JAK2 (V617F), and a typical bone marrow biopsy. Three years later, this woman developed erythrocytosis: serum erythropoietin was low at this time whereas the granulocyte JAK2 (V617F)-mutant allele burden was increased, indicating a polycythemic transformation. We did not repeat bone marrow biopsy because a diagnosis of PV could be made otherwise according to the British Committee for Standards in Hematology (BCSH) criteria,32 and more importantly because a phlebotomy program was already indicated. JAK2 (V617F)-mutant ET has a cumulative risk of polycythemic transformation equal to 29% at 15 years.10 We check the JAK2 (V617F)-mutant allele burden during follow-up in all patients who show an increase in hemoglobin level above the upper normal limit.
Case 4: High-risk JAK2-mutant ET
The diagnostic process was simple in this man. He had normal body iron status and no evidence of inflammation, and more importantly he carried JAK2 (V617F). This case is reported mainly to emphasize the concept that advanced age represents a risk factor for thrombosis per se.
Case 5: MPL-mutant MDS/MPN-RS-T mimicking ET
The combination of mild anemia and thrombocytosis suggested MDS/MPN-RS-T: this diagnosis was confirmed by the examination of bone marrow aspirate and biopsy, including Perls staining. No SF3B1 mutation was detected in this woman, as happens in about one-third of these patients. This case illustrates the importance of performing bone marrow aspirate and biopsy to make an accurate morphologic diagnosis of ET and to distinguish this disorder from other myeloid neoplasms. To this purpose, evaluation of bone marrow biopsy should always include Perls and Gomori staining: without Perls staining, this patient might have been misdiagnosed as MPL-mutant ET.
How we communicate diagnosis of ET
We inform our patients that ET belongs to MPNs and that these are disorders of the bone marrow characterized by excessive production of peripheral blood cells. We underline that although ET is a tumor (that is, a neoplasm), this is a chronic disorder with a minimal impact on the patient’s life expectancy.33 We also explain that ET is an acquired disorder and that the driver mutation (in JAK2, CALR, or MPL) is always somatically acquired. Although familial cases exist,34,35 likely caused by a genetic predisposition to acquire the above somatic mutations, we emphasize that ET cannot be directly inherited by the progeny. We suggest screening of relatives only in familial trees with 2 or more subjects affected with MPNs (see “Distinguishing familial ET from hereditary thrombocytosis”).
We then illustrate the 3 most important complications that can occur during follow-up10,36 : (1) vascular complication (15-year cumulative risk of thrombosis ranging from 10% to 25% depending on the molecular subtype, higher in JAK2-mutant than in CALR-mutant ET)10 ; (2) progression to myelofibrosis (15-year cumulative risk of about 10% on average, higher in type 1 CALR-mutant than in JAK2-mutant ET)37 ; and (3) leukemic transformation (15-year cumulative risk of about 3% on average).10
Very often, patients ask whether they should modify their dietary habits, physical activity, or their lifestyle in general. We recommend continuing a normal life without deprivation of food, sports, and enjoyment, just taking into account that antiplatelet therapy may expose the patient to bleeding in the case of extreme sports. We advise stopping smoking and recommend treating obesity, hypertension, and dyslipidemia. Finally, we discuss the importance of an optimal follow-up. We suggest checking CBC count regularly, monthly if a cytoreductive treatment is started, and planning a hematologic visit every 6 months. When the condition is stable, CBC counts can be checked less frequently. Abdominal ultrasound is recommended when myelofibrotic transformation is suspected based on clinical and/or hematologic criteria.
How we treat patients with ET according to their individual risk
Currently available treatments for ET patients are mainly aimed at minimizing the risk of thrombosis and/or bleeding. As shown in Table 4, age ≥60 years, history of vascular complications (thrombosis or major bleeding), and PLT count ≥1500 × 109/L are the 3 risk factors used to classify patients with ET into low (no risk factors) and high risk (1 or more risk factors).38 A PLT count ≥1500 × 109/L represents an indication to cytoreductive treatment because extreme thrombocytosis is frequently associated with acquired von Willebrand syndrome.38 The International Prognostic Score for Essential Thrombocythemia (IPSET) is based on age >60 years, white blood cell (WBC) count >11 × 109/L, and history of thrombosis3 : although it provides prognostic information, we do not use it for clinical decision-making at present.
As illustrated in Table 4, in our current practice we treat low-risk patients with low-dose aspirin or just follow them regularly; we treat high-risk patients with low-dose aspirin combined with a cytoreductive treatment.
According to the European LeukemiaNet (ELN) recommendations,38 all ET patients should be managed with low-dose aspirin if microvascular disturbances are present. A study conducted on low-risk ET patients showed that antiplatelet therapy reduces the incidence of venous thrombosis in JAK2-mutated patients and the rate of arterial thrombosis in those with cardiovascular risk factors.39 In the remaining low-risk patients, antiplatelet therapy was not effective as primary prophylaxis of thrombosis. Subsequent studies have confirmed that cardiovascular risk factors and JAK2 (V617F) represent independent risk factors for thrombosis in ET.4,40,41 A more recent work has evaluated the benefit-to-risk ratio of low-dose aspirin in 433 low-risk ET patients who were on antiplatelet therapy or observation only.42 In JAK2 (V617F)-mutated patients, low-dose aspirin was associated with a reduced incidence of venous thrombosis with no effect on the risk of bleeding. By contrast, in CALR-mutated patients, antiplatelet therapy did not affect the risk of thrombosis whereas it was associated with a higher incidence of bleeding. Thus, the available evidence indicates that JAK2 (V617F) plays a major role in the pathogenesis of thrombosis, whereas CALR or MPL mutation and triple negativity identify patients with lower thromboembolic risk.
Based on the available evidence, we use low-dose aspirin in ET patients as summarized in Table 4. We prescribe aspirin 100 mg once daily, preferably at the end of a meal, and do not try to modulate the dosing interval as suggested by a study on thromboxane biosynthesis.43 We administer antiplatelet therapy to all high-risk ET patients with the only exception being those with extreme thrombocytosis (≥1500 × 109/L). In low-risk patients, we base our decision on the molecular subtype and the presence of cardiovascular risk factors or microvascular symptoms (Table 4). In case of marked thrombocytosis (≥1000 × 109/L) or of any evidence of bleeding, before starting low-dose aspirin, we assess von Willebrand factor in terms of antigen level and ristocetin cofactor activity to exclude an acquired von Willebrand syndrome; if present, we do not administer aspirin. In case of gastric intolerance, we suggest taking antacid drugs or switching to ticlopidine.
Although cytoreductive treatment is not indicated in low-risk ET patients who have an incidence of thrombosis similar to that of a healthy control population,44 it is definitely indicated in high-risk patients.45,46 Our criteria for the choice of the optimal cytoreductive drug are reported in Table 5.
The ELN experts recommended hydroxyurea as first-line cytoreductive therapy at any age, although they also underlined that its use should be carefully considered in patients younger than 40 years of age.38 In our current practice, we start hydroxyurea at a dose of 1 g per day, and then modify the dose according to the hematologic response. The objective of treatment is dual, that is: (1) to lower PLT count below 450 × 109/L and (2) to correct leukocytosis (WBC count ≥11 × 109/L), if present. We consider correcting leukocytosis very important, whereas we are relatively flexible with respect to the PLT count: actually, we consider acceptable also values between 450 × 109/L and 600 × 109/L, especially if these values can be achieved with lower doses of hydroxyurea (eg, 500 mg per day). Our clinical practice is supported by a number of studies showing that the actuarial probability of thrombosis is influenced by leukocytosis and not by PLT count.47-50 In the prospective Thrombocythemia 1 Trial (PT-1) cohort, PLT count outside of the normal range during follow-up was not associated with a risk of thrombosis, but rather with a risk of bleeding.50
In patients younger than 40 years of age and in selected cases between 40 and 60 years of age (Table 5), we use interferon α: this represents an off-label use because ET is not an approved indication. Although there is no evidence that hydroxyurea can increase the risk of leukemic transformation,51 this drug is not completely devoid of adverse effects52 ; more importantly, it does not specifically target the mutant clone and is therefore unlikely to modify the natural history of disease. On the contrary, pegylated interferon α-2a has been shown to induce sustained complete molecular response in a subset of patients with JAK2 (V617F)-mutant ET.53,54 In addition, recent reports describe the positive effect of interferon α in patients with CALR-mutant ET16,55 : this treatment was found to produce high rates of hematologic and molecular responses that in some cases could be maintained after discontinuation of the drug.55 Although these observations do not necessarily mean that interferon α can modify the natural history of disease, they at least indicate that this drug can target the myeloproliferative clone.56
Before starting treatment with interferon α, we suggest evaluating liver and thyroid function, and excluding the presence of autoantibodies (anti-nuclear and anti-double-stranded DNA antibodies, rheumatoid factor, and anti-neutrophil cytoplasmic antibodies). We generally start with 3 × 106/million units of interferon α 3 times a week, and then titrate the dose according to efficacy and side-effects. To avoid flu-like symptoms, we recommend the use of paracetamol, 1000 mg per os, at the beginning of treatment. Adverse effects of interferon α treatment are not trivial, and include, besides flu-like symptoms, nausea, fatigue, and psychiatric sequelae.57 Previous studies have indeed reported discontinuation of treatment in almost one-fourth of cases.58 Patients must therefore be supported and motivated, underlying that this represents so far the only treatment capable of targeting the mutant cells in their bone marrow.
The PT-1 trial showed that hydroxyurea plus low-dose aspirin is superior to anagrelide plus low-dose aspirin for patients with ET at high risk for vascular events.59 More recently, the ANAHYDRET study concluded that anagrelide is not inferior compared with hydroxyurea in the prevention of thrombotic complications in patients with ET diagnosed according to the 2008 WHO criteria.7 The US Food and Drug Administration (FDA) has approved anagrelide for the treatment of ET, whereas the European Medicine Agency (EMA) has been more restrictive, approving the drug for treatment of patients with high-risk ET who are intolerant to their current therapy or whose elevated PLT counts are not reduced to an acceptable level by their current therapy. An expert panel has provided a definition of clinical resistance/intolerance to hydroxyurea in ET.60 So far, we have only occasionally considered the use of anagrelide, mainly because the number of ET patients with resistance/intolerance to hydroxyurea is small in our experience. However, we are fully aware that anagrelide is widely used alone and in combination in other institutions.61 Because of the potential cardiovascular toxicity associated with anagrelide, especially in older patients, a careful cardiac evaluation is recommended before starting treatment.
Busulfan and pipobroman have been used as second-line treatment in older patients who are unresponsive or intolerant to hydroxyurea. We currently use busulfan to this purpose: before prescribing this drug, we inform the patient that alkylating agents and the sequential use of different cytoreductive treatments might increase the risk of leukemic transformation.36,51,62 Microvascular symptoms such as erythromelalgia and acroparesthesia generally resolve with low-dose aspirin. We consider an ad hoc cytoreductive treatment only in the few patients whose symptoms severely affect quality of life and do not respond to low-dose aspirin.
Clinical vignettes: defining the individual risk and deciding therapy
Clinical vignettes are explored in detail in Table 1.
Case 1: Low-dose aspirin in low-risk JAK2-mutant ET
This patient had low-risk JAK2 (V617F)-mutant ET. She was given low-dose aspirin (100 mg per day) because JAK2 (V617F) specifically involves a high risk of thrombosis.10 This patient is currently seen a couple of times a year in the Outpatient Department.
Case 2: Low-risk CALR-mutant ET with acquired von Willebrand syndrome
This woman had CALR-mutant ET and a history of oral mucosal bleeding after tooth brushing. Because of marked thrombocytosis (PLT count, 1069 × 109/L), we assessed von Willebrand factor and found reduced ristocetin cofactor activity (30%) associated with normal antigen level, a combination that indicates an acquired von Willebrand syndrome. We decided on a watchful-waiting strategy: cytoreductive treatment with interferon α would be considered if PLT count increases to ≥1500 × 109/L and/or bleeding becomes more severe.
Case 3: JAK2-mutant ET requiring phlebotomies because of progression to PV
This woman had low-risk JAK2 (V617F)-mutant ET and therefore we prescribed low-dose aspirin. She then progressed to PV and a phlebotomy program to maintain hematocrit <45% was started. Her subsequent clinical course has been characterized by a progressive increase in PLT count due to the iron deficiency caused by phlebotomies. If PLT count increases to ≥1500 × 109 and/or leukocytosis develops, we would discontinue phlebotomies and start a cytoreductive treatment with hydroxyurea.
Case 4: Low-dose aspirin and hydroxyurea in high-risk JAK2-mutant ET
This man was over 60 years of age and therefore had high-risk JAK2 (V617F)-mutant ET. He was treated with low-dose aspirin and hydroxyurea (1 g per day): PLT count normalized quickly and remained steadily below 300 × 109/L after the dose of hydroxyurea was reduced to 500 mg per day. This optimal response to hydroxyurea is not uncommon in patients with JAK2 (V617F)-mutant ET.
Case 5: Lack of evidence regarding treatment of a rare myeloid neoplasm
MDS/MPN-RS-T is a very rare disease and the available evidence regarding its treatment is limited. We do not use cytoreductive drugs in these patients because they might significantly worsen anemia and/or negatively impact on the risk of leukemic transformation. Low-dose aspirin was given to this 64-year-old woman for primary prevention of thromboembolic complications.
Distinguishing familial ET from hereditary thrombocytosis
Case 6 in Table 6 illustrates our approach to the identification of familial cases of ET.
Familial thrombocytosis includes hereditary thrombocytosis and familial ET. Hereditary thrombocytosis may be associated with germ line mutations of THPO, the thrombopoietin gene,63,64 or MPL, the thrombopoietin receptor gene.23,24,65 Recent reports have described cases of hereditary thrombocytosis associated with noncanonical germ line mutations of JAK2.23,66-69 In familial ET, JAK2 (V617F) is always a somatically acquired event, as in the familial tree reported in Figure 2.34,70,71
Familial ET. In this family, JAK2 (V617F) was a somatically acquired mutation found in circulating granulocytes (with variable values for mutant allele burden in the different patients) but not in circulating T lymphocytes. Familial ET must be distinguished from hereditary thrombocytosis, a Mendelian genetic disease attributable to germ line mutations of JAK2, MPL, or THPO.
Familial ET. In this family, JAK2 (V617F) was a somatically acquired mutation found in circulating granulocytes (with variable values for mutant allele burden in the different patients) but not in circulating T lymphocytes. Familial ET must be distinguished from hereditary thrombocytosis, a Mendelian genetic disease attributable to germ line mutations of JAK2, MPL, or THPO.
In our clinical practice, we interview all patients with thrombocytosis to find out if there is a family history of thrombocytosis or MPN. In familial trees with at least 2 cases of MPN, we suggest performing a CBC count in all apparently healthy relatives with the aim of identifying an early asymptomatic MPN phenotype. We manage and treat patients with familial ET in the same way as patients with sporadic ET; a recent study found survival to be similar in familial and sporadic MPN patients.72
In familial trees with suspected hereditary thrombocytosis, we now sequence the entire coding region of JAK2 and MPL as well as the THPO gene.
How we manage pregnancy in women with ET
Case 7 in Table 6 provides an example of how we manage pregnancy in a woman with ET.
ET occurs in women of childbearing age and pregnancy is a relatively common issue in the clinical management of young women with this disorder. Previous studies have shown live birth rates of 50% to 70%, and spontaneous abortion rates of 25% to 50%, mostly during the first trimester.73 The pathogenesis of these complications is unclear; age, parity, thrombophilia, PLT count, WBC count, and hemoglobin level have not been found to be predictive of pregnancy outcome in ET.74-76 Whether the use of aspirin can improve pregnancy outcome is uncertain74-77 ; however, a meta-analysis of randomized trials conducted outside ET concluded that low-dose aspirin is effective in preventing preeclampsia, being safe for both mother and fetus.78 Pregnancy complications in women with ET are associated with a higher risk of subsequent thrombosis.79
In our practice, we inform the patient and her partner that pregnancy is not discouraged in ET, although they should be aware that the risk of fetal loss is 3 times higher compared with that of healthy women. We recommend aspirin for all pregnant women with ET, unless contraindicated. If PLT count is ≥1000 × 109/L, an acquired von Willebrand syndrome should be excluded as indicated before. In agreement with the ELN recommendations, we add low-molecular-weight heparin (LMWH) to low-dose aspirin in case of previous major thrombosis or severe pregnancy complication, and consider interferon α if the PLT count is ≥1500 × 109/L or in case of previous major bleeding.38
We discuss with obstetricians and anesthetists the optimal time to discontinue antiplatelet treatment (generally about 2 weeks before delivery) in order to account for any possible event, including instrumental delivery and epidural or spinal anesthesia. After delivery, we recommend treating all women with ET with LMWH for 6 weeks to prevent deep vein thrombosis.79
Returning to case 7, we used a combination of low-dose aspirin and LMWH as this pregnancy was classified as high risk because of the previous history of severe preeclampsia with preterm delivery.
Potentially catastrophic events: how we manage SVT in patients with ET
Case 8 in Table 6 provides an example of how we manage splanchnic vein thrombosis (SVT) in patients with ET.
MPNs are the leading cause of SVT, being responsible for about half of cases of Budd-Chiari syndrome and one-third of cases of portal vein thrombosis.80 In a meta-analysis of 831 patients with SVT, the mean prevalence of positivity for JAK2 (V617F) was 32.7%81 : because this may be the first marker of a latent MPN, we recommend routine screening for JAK2 (V617F) in patients with a SVT. Although the incidence of CALR mutations is low in patients with SVT,82 we have now also included mutation analysis of CALR exon 9 in the workup of these subjects.
Genetic and acquired thrombophilia, in particular the presence of an antiphospholipid syndrome, should be studied in all cases of ET with SVT. Because ET patients with genetic or acquired thrombophilia are at high risk of recurrent thrombosis,83 we prescribe cytoreductive treatment (generally hydroxyurea) and oral anticoagulation through life. These patients should be regularly monitored for portal hypertension and esophageal varices.
Resistance to conventional treatments and the use of experimental drugs
Case 9 in Table 6 provides an example of the efficacy of ruxolitinib in a patient with ET who was resistant to conventional treatments, including interferon α and hydroxyurea.
In a phase 2 open-label study,84 ruxolitinib treatment was well tolerated in a population of patients with ET who were refractory or intolerant to hydroxyurea, and resulted in improvements in PLT count and disease-related symptoms in most cases. Interestingly, 2 patients with JAK2 (V617F)-mutant ET enrolled in this study achieved complete molecular remission after 5 years of ruxolitinib treatment.85 It should also be noted that patient 8 (Table 6) had a complete hematologic response to ruxolitinib after enrollment in a clinical trial on the use of this JAK inhibitor for treatment of SVT associated with MPNs.
In a phase 2, open-label study, the telomerase inhibitor imetelstat has been administered to 18 patients with ET who had not had a response to or who had unacceptable side-effects from prior therapies.86 This study showed hematologic and molecular responses but also nonnegligible adverse events. It has been suggested that imetelstat may change the natural history of MPN,87 but its side-effect profile appears hardly acceptable in ET patients.
Conclusions and perspectives
Although our understanding of the genetic basis of ET has improved considerably in the last few years, there has been less progress than we would have liked in the management of this disorder. Low-dose aspirin combined with hydroxyurea may represent a satisfactory treatment of patients over the age of 60 years, but there is a need of disease-modifying drugs for younger patients. Current areas of uncertainty in the management of ET patients are reported in Table 7.
Table 7 also includes a list of potential disease-modifying drugs. The ongoing randomized trial of pegylated interferon α-2a vs hydroxyurea in PV and ET (ClinicalTrials.gov Identifier: NCT01259856) may provide important information about the ability of this drug to modify the natural history of disease. Ropeginterferon α-2, a monopegylated interferon α-2b isoform that can be administered every 2 weeks, has been recently shown to be safe and effective in the treatment of PV88 : in this study, two-thirds of patients showed a reduction in the JAK2-mutant allele burden during treatment. Whether JAK inhibitors can provide beneficial effects in patients with ET remains to be established in ad hoc clinical trials, but ruxolitinib has already proved to be effective in patients with a relatively aggressive disease.84
Acknowledgments
The authors thank their patients with MPNs for participating in their studies. Most of their studies on MPNs are currently done within the Associazione Italiana per la Ricerca sul Cancro (AIRC)–Gruppo Italiano Malattie Mieloproliferative project. The current investigations are funded by the following grants from AIRC, Milan, Italy: MFAG 2014 Id.15672; and Special Program Molecular Clinical Oncology 5×1000, #1005.
Authorship
Contribution: E.R. and M.C. conceived and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisa Rumi, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: elisa.rumi@unipv.it; or Mario Cazzola, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: mario.cazzola@unipv.it.