In this issue of Blood, Lichtenstein et al1 report a recurring mutation in NDUFB11, a mitochondrial respiratory complex I–associated protein encoded on the X chromosome, in 5 males with congenital sideroblastic anemia (CSA). The mutation resulted in respiratory insufficiency and loss of complex I stability and activity in patient-derived fibroblasts. This finding corroborates other evidence suggesting that sideroblastic anemia can be caused by dysfunction of the mitochondrial respiratory chain (RC).
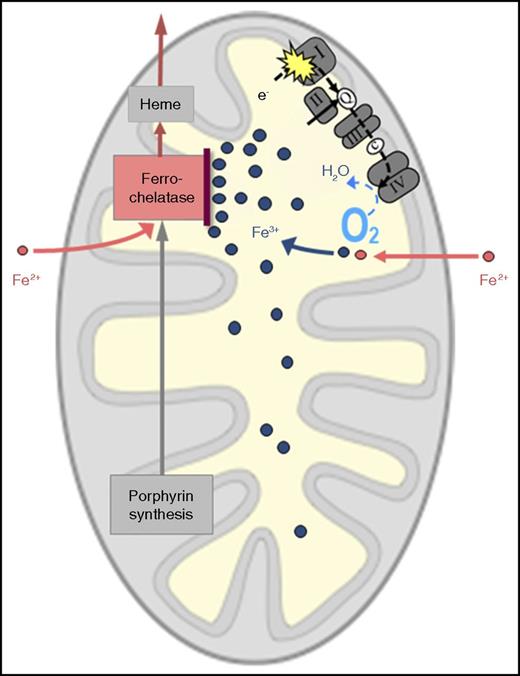
Probable mechanism of RC dysfunction causing mitochondrial iron overload. I, II, III, and IV represent multienzyme complexes of the RC. c, cytochrome c; e-, electron(s); Q, coenzyme Q.
Probable mechanism of RC dysfunction causing mitochondrial iron overload. I, II, III, and IV represent multienzyme complexes of the RC. c, cytochrome c; e-, electron(s); Q, coenzyme Q.
The first evidence of mitochondrial RC involvement in sideroblastic anemia came from Pearson’s syndrome, a rare congenital disorder characterized by lactic acidosis, pancreatic exocrine insufficiency, and sideroblastic anemia. The disease is caused by large deletions of mitochondrial DNA (mtDNA).2 Because all 13 proteins encoded by the mitochondrial genome are components of the RC, large mtDNA deletions lead to impaired mitochondrial respiration. RC defects can also result from altered mitochondrial transfer RNAs (tRNAs) disturbing mitochondrial protein synthesis. Such a mechanism seems to be involved in CSAs caused by mutation of the mitochondrial tyrosyl-tRNA synthetase gene (YARS2),3 mutation of tRNA nucleotidyl transferase 1 (TRNT1),4 or mutation of pseudouridine synthase 1 (PUS1).5
Another possible cause of RC malfunction is impaired mitochondrial biogenesis of iron–sulfur clusters (ISCs), because the RC, in particular complex I, contains iron–sulfur proteins that are critical for electron transport. This may partly explain congenital sideroblastic anemia caused by mutation of glutaredoxin 5, which is involved in mitochondrial ISC assembly.6
The reversible sideroblastic anemia of copper deficiency can also be explained in terms of RC dysfunction, because copper is pivotal to the functioning of RC complex IV (cytochrome c oxidase).
In all these circumstances, the following question arises: how does respiratory insufficiency lead to mitochondrial iron overload? The following mechanism appears plausible (see figure). As the RC normally consumes most of the oxygen that reaches the mitochondrion, a defective RC, by decreasing O2 consumption, will leave more O2 than usual in the mitochondrial matrix. This can lead to oxidation of the incoming iron, which has crossed the inner mitochondrial membrane in the divalent form (ferrous iron, Fe2+) and must remain in the divalent form to be accepted by ferrochelatase for incorporation into protoporphyrin IX to make heme. If surplus oxygen converts Fe2+ to the trivalent form (ferric iron, Fe3+), this “rusted iron” is useless for heme synthesis, because it is rejected by ferrochelatase and will thus accumulate in the mitochondrial matrix.
Does this pathogenetic mechanism also play a role in the most common form of sideroblastic anemia, namely that related to myelodysplastic syndromes (MDS)? Acquired, clonally amplified mutations of mtDNA have been identified in MDS,7 but circumstantial evidence of a causative relation with the sideroblastic phenotype and/or ineffective erythropoiesis has only been provided in a few cases.8,9 Matthes and coworkers confirmed RC dysfunction in a case of CSA with large mtDNA deletion but were unable to find RC dysfunction in 5 patients with refractory anemia with ringed sideroblasts (RARS).10 It is tempting to speculate that acquired mutations (in mtDNA or nuclear DNA) that cause severe RC dysfunction are incompatible with incessant clonal proliferation in MDS and are therefore selected against. In MDS-related sideroblastic anemia, the mechanism should thus be sought outside the RC in most cases. This is in line with the finding that ∼80% of patients with RARS or refractory cytopenia with multilineage dysplasia and ringed sideroblasts carry an acquired mutation of SF3B1, a component of the splicing machinery. Although we know that a wide range of pre–messenger RNAs, including that of the mitochondrial transporter ABCB7, are aberrantly spliced and undergo nonsense-mediated decay, we do not yet understand how this leads to mitochondrial iron overload. Lichtenstein et al have identified an interesting cause of CSA, and future analyses of families with CSA may reveal further defects of the RC. For MDS, however, the riddle of the ring sideroblast remains to be solved.
Conflict-of-interest disclosure: The author declares no competing financial interests.