Key Points
Blockade of ST2 improves morbidity and mortality in murine FHL.
Danger signals such as IL-33 may be required to amplify antigen-specific immune responses above the threshold for FHL disease in mice.
Abstract
Cytokine storm syndromes, such as familial hemophagocytic lymphohistiocytosis (FHL), are lethal disorders caused by uncontrolled, systemic immune activation. In the murine model of FHL, in which perforin-deficient (Prf1−/−) mice are infected with lymphocytic choriomeningitis virus (LCMV), disease is driven by overabundant interferon (IFN)γ-producing LCMV-specific CD8+ T cells thought to arise from excessive antigen stimulation through the T-cell receptor. However, this paradigm is insufficient to explain several fundamental aspects of FHL, namely, the inability of many pathogenic antigens to induce hyperinflammation, and the previously identified role of MyD88 in the disease. We now show a novel role for the MyD88-dependent interleukin-33 (IL-33) receptor, ST2, in FHL. Expression of IL-33 and ST2 is upregulated in LCMV-infected Prf1−/− mice. Blockade of ST2 markedly improves survival of LCMV-infected Prf1−/− mice and reduces the severity of multiple disease parameters, including serum levels of IFNγ. This decrease in IFNγ corresponds to a reduction in both the frequency of IFNγ+ LCMV-specific CD8+ and CD4+ T cells and the magnitude of IFNγ expression in these cells. These findings demonstrate that disruption of ST2 signaling in the murine model of FHL reduces T cell–mediated production of IFNγ and suggest a revised paradigm in which danger signals such as IL-33 are crucial amplifiers of immune dysregulation in FHL. Furthermore, this study provides evidence to support blockade of ST2 as a novel therapeutic strategy for FHL.
Introduction
Hemophagocytic syndromes are life-threatening, cytokine-driven disorders associated with a wide range of disease states and are increasingly recognized as a significant clinical problem. The primary form of hemophagocytic syndrome, known as familial hemophagocytic lymphohistiocytosis (FHL), is caused by genetic defects in perforin (FHL type 2, or FHL2) or other proteins in the granule exocytosis pathway.1,2 Because of the absence of immune-mediated cytotoxicity in FHL, viral infections and other inflammatory stimuli trigger an ineffective yet hyperactive immune response leading to fatal immunopathology.3 The difficulty in treating FHL and related hemophagocytic syndromes stems from a paucity of effective therapies and an incomplete understanding of the underlying pathophysiology.
In the FHL2 murine model, perforin-deficient (Prf1−/−) mice infected with lymphocytic choriomeningitis virus (LCMV) develop lethal inflammation driven by overabundant interferon (IFN)γ-producing LCMV-specific CD8+ T cells.4-6 Prior studies have attributed this hyperactive T-cell response to excessive antigen stimulation through the T-cell receptor (TCR),7 which is due to the inability to eliminate antigen-presenting cells. Although this mechanism accounts for part of the disease phenotype, it fails to explain why persistent antigen in the context of infection is not always sufficient to induce FHL in murine models and in patients. Of the numerous viruses tested in Prf1−/− mice, only LCMV and murine cytomegalovirus are documented to cause hemophagocytic syndrome.2 Moreover, a portion of FHL patients present with hemophagocytic syndrome only later in childhood or adulthood, by which time they have certainly experienced multiple viral infections.8 Together, these observations suggest that additional unidentified factors are required for the development of FHL.
Given the importance of pathogen- and danger-associated molecular patterns in initiating inflammation, a previous study focused on the adaptor protein MyD88, which is required for signaling by interleukin (IL)-1 family cytokines and most Toll-like receptors (TLRs). Using the murine model of FHL type 3 (FHL3), in which Unc13djinx/jinx mice are infected with LCMV, this study demonstrated that loss of MyD88 signaling confers protection from hemophagocytic syndrome.9 Unc13djinx/jinx/Myd88poc/poc mice developed LCMV-specific CD8+ T-cell frequencies comparable to those of wild-type (WT) mice, suggesting that rather than limiting the ability of antigen-presenting cells to prime T-cell responses, loss of MyD88 signaling abrogated a crucial proinflammatory signal.9 These data demonstrate a requirement for additional MyD88-dependent, antigen-independent signals for disease induction, but it remains unclear which mediators upstream of MyD88 are responsible for promoting the development of FHL.
In this study, we investigated the role of MyD88-dependent signaling pathways in precipitating disease in FHL2 mice, with a particular focus on the IL-1 receptor family member ST2 and its ligand, IL-33. IL-33 is constitutively expressed in the nuclei of nonhematopoietic cells and is expelled upon cellular stress or necrosis.10-12 When it is released to the extracellular space, IL-33 signals a diverse range of immune cells that express its receptor (ST2/IL-1RAcP complex).13,14 IL-33 is thus classified as an alarmin, in that it activates an inflammatory response in the context of tissue damage.11,15,16 We ultimately identify ST2 as a novel factor promoting FHL. Our data demonstrate that ST2 signaling enhances CD8+ and CD4+ T-cell IFNγ overproduction, leading to hypercytokinemia and fatal disease, and suggest IL-33/ST2 as a promising therapeutic target. Furthermore, our work provides evidence for revising the traditional model of FHL pathophysiology to take into account danger signals derived from tissue damage.
Materials and methods
Mice
C57BL/6 (WT) and perforin-deficient (C57BL/6-Prf1tm1Sdz/J, referred to as Prf1−/−) mice were purchased from The Jackson Laboratory and bred in our facility. Myd88−/− mice were a kind gift from Lawrence Turka (The Children’s Hospital of Philadelphia) and were crossed to Prf1−/− mice.17 Il33−/− mice were provided by Amgen.18 All animal studies were performed with the approval of The Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee.
Induction of FHL2
Mice aged 7 to 9 weeks were infected intraperitoneally with 2 × 105 plaque-forming units of LCMV-Armstrong strain and were euthanized upon development of significant morbidity or weight loss. Peripheral blood was obtained by cheek bleed, and complete blood cell counts were performed on a Hemavet analyzer (Drew Scientific). Serum ferritin (ALPCO), soluble CD25 (R&D Systems), and IFNγ (BD Biosciences) were measured using enzyme-linked immunosorbent assay. Viral titers were measured by plaque assays on Vero cells as previously described.19
Quantitative real-time polymerase chain reaction
RNA was isolated from RNAlater-preserved tissues using the RNeasy Mini kit (Qiagen), converted to complementary DNA using the Superscript III First-Strand Synthesis System (Life Technologies), and subjected to quantitative real-time polymerase chain reaction using QuantiTect primers for Actb, Il33, and Il1rl1 (Qiagen) and Power SYBR Green master mix (Life Technologies). Results were normalized to β-actin using the ΔΔCT method.
Histology and immunohistochemistry
Unperfused organs were fixed overnight in 4% paraformaldehyde and embedded in paraffin. Liver and spleen sections were stained with hematoxylin and eosin. Deparaffinization, retrieval, and immunohistochemistry were done on the Leica Bond III Autostainer using the Bond Polymer Refine Detection System (Leica Microsystems). IL-33 goat primary antibody (R&D Systems) was run at a 1:30 dilution after epitope retrieval with ER2 buffer (Leica), and rabbit anti-goat secondary antibody (Jackson ImmunoResearch Labs) was added. Slides were read by pediatric pathologists (M.P., P.A.K.) blinded to treatment protocols. Images were acquired on an Eclipse 90i microscope (Nikon, Melville, NY) using an ×20 (NA 0.75) Plan Apochromatic objective and NIS Elements BR 4.13.04 software.
Analysis of human gene expression
In vivo ST2 blockade
Rat anti-mouse ST2-blocking antibody with muIgG1 Fc domain (α-ST2 antibody) and mouse IgG1 isotype control antibody were provided by Amgen and have been previously described.23 LCMV-infected mice were injected intraperitoneally with 150 μg of α-ST2 antibody or 150 μg of control antibody every other day, beginning on day 3 postinfection.
Flow cytometric analysis
Splenocytes and intrahepatic leukocytes were stained with LIVE/DEAD fixable viability dye (Life Technologies) and CD4, CD8α, CD44, CD62L, CD90.2, and/or CD127 antibodies (BD Pharmingen, eBioscience, BioLegend, and Miltenyi Biotec). H-2DbGP33-41 and I-AbGP66-77 major histocompatibility complex–peptide complexes were provided as fluorophore-conjugated tetramers by E.J.W. and the National Institutes of Health Tetramer Core Facility, respectively. All samples were acquired on a MACSQuant flow cytometer (Miltenyi Biotec) and analyzed using FlowJo software version 9.8 (Tree Star).
Intracellular cytokine staining
Splenocytes (106) were cultured in the absence or presence of 0.2 μg/mL LCMV gp33 peptide (GenScript) or 1.0 μg/mL LCMV gp61 peptide (Anaspec) and brefeldin A (Sigma-Aldrich) for 5 hours at 37°C. After staining with LIVE/DEAD and for surface antigens as described earlier, cells were stained for IFNγ (clone XMG1.2) using the Cytofix/Cytoperm kit (BD Bioscience). The frequency of IFNγ+ LCMV-specific cells was calculated as (%IFNγ+ with peptide) − (%IFNγ+ without peptide).
Statistical analysis
Weight loss data and T-cell data were analyzed by linear mixed-effects models using R (R Core Team, 2014) and lme4.24 Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. P values were obtained by likelihood ratio tests of the full model with the effect in question against the model without the effect in question. We used the method of Levy25 to obtain tests of main effects while modeling an interaction effect. All other data were analyzed in GraphPad Prism 5 using statistical tests indicated in the figure legends.
Results
MyD88 is required for the development of FHL2 in mice
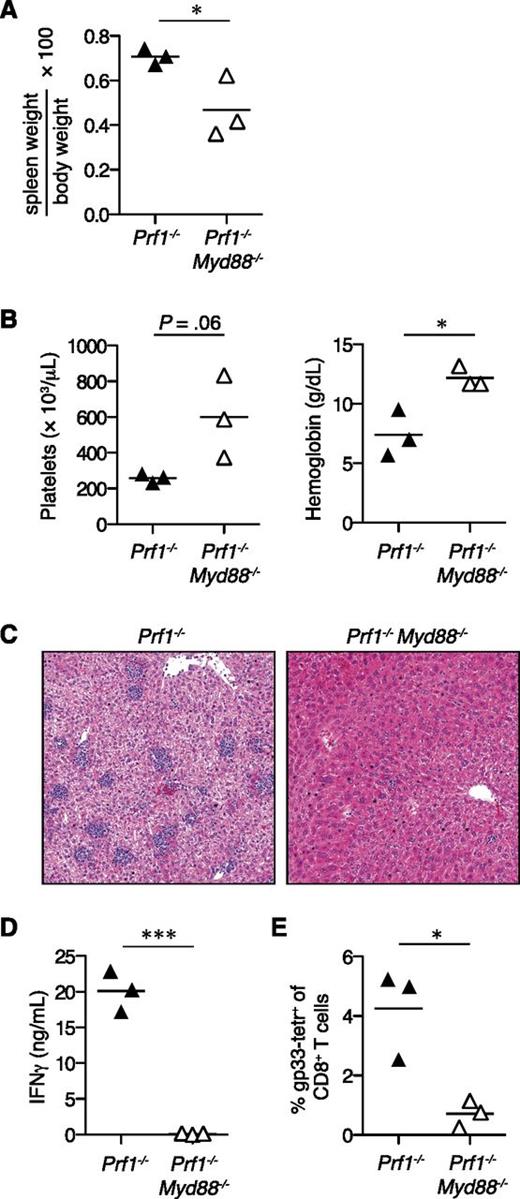
MyD88 is necessary for disease in the murine model of FHL3.9 To determine whether MyD88 signaling contributes to FHL2, we compared the response of Prf1−/− and Prf1−/−Myd88−/− mice to LCMV infection. Prf1−/− mice became moribund by day 10 postinfection, whereas Prf1−/−Myd88−/− mice did not (data not shown). MyD88 deficiency protected Prf1−/− mice from multiple FHL disease parameters, including splenomegaly, anemia, and thrombocytopenia (Figure 1A-B). Hepatitis was also markedly reduced in Prf1−/−Myd88−/− mice, because these mice demonstrated considerably fewer lobular foci of inflammatory infiltrates compared with Prf1−/− controls (Figure 1C). Consistent with their reduced FHL severity, Prf1−/−Myd88−/− mice had decreased levels of serum IFNγ and frequencies of CD8+ T cells specific for the immunodominant LCMV epitope gp33 compared with Prf1−/− mice (Figure 1D-E). These results suggest that non-TCR signaling pathways such as MyD88 are important for promoting disease in the FHL2 murine model, similar to findings in the FHL3 model.
MyD88 is required for the development of FHL2 in mice.Prf1−/− mice (n = 3) and Prf1−/−Myd88−/− mice (n = 3) were infected with LCMV and analyzed 10 days postinfection for signs of FHL2. Representative of 3 independent experiments. Analyzed by Student 2-tailed t test. (A) Spleen weight expressed as a ratio of total body weight. (B) Platelet counts and hemoglobin levels from peripheral blood. (C) Representative hematoxylin and eosin–stained liver sections, original magnification ×100. (D) Serum IFNγ levels. (E) Frequencies of splenic LCMV-specific T cells stained with gp33 major histocompatibility complex class I tetramer (tetr). *P < .05; ***P < .001.
MyD88 is required for the development of FHL2 in mice.Prf1−/− mice (n = 3) and Prf1−/−Myd88−/− mice (n = 3) were infected with LCMV and analyzed 10 days postinfection for signs of FHL2. Representative of 3 independent experiments. Analyzed by Student 2-tailed t test. (A) Spleen weight expressed as a ratio of total body weight. (B) Platelet counts and hemoglobin levels from peripheral blood. (C) Representative hematoxylin and eosin–stained liver sections, original magnification ×100. (D) Serum IFNγ levels. (E) Frequencies of splenic LCMV-specific T cells stained with gp33 major histocompatibility complex class I tetramer (tetr). *P < .05; ***P < .001.
Splenic expression of IL-33 is enhanced in FHL2 mice, and expression of ST2 is increased in both mice and patients with FHL2
To delineate the signaling mediators upstream of MyD88 that contribute to the development of FHL, we focused on signaling through IL-1 family receptors. Although in vivo blockade of either IL-1 or IL-18 signaling has no effect on FHL mortality,4,9 the role of IL-33 signaling in FHL has not previously been investigated. Recent studies have shown that ST2 deficiency in LCMV-infected mice leads to defective expansion and polyfunctionality of LCMV-specific CD8+ and CD4+ T cells, as well as protection from mortality in a CD8+ T cell-mediated model of immunopathology.26,27 These findings led us to hypothesize that ST2 signaling upstream of MyD88 contributes to FHL inflammation.
We first determined whether IL-33 and ST2 are expressed in the organs most affected by FHL in LCMV-infected Prf1−/− mice (referred to as FHL2 mice). Although hepatic expression of Il33 remained stable in both WT and Prf1−/− mice after LCMV infection, splenic Il33 was greatly upregulated (Figure 2A). Notably, Il33 expression was highest in the spleens of FHL2 mice. Immunohistochemical analysis confirmed nuclear localization of IL-33 in WT and Prf1−/− livers and spleens (Figure 2B and data not shown). Although the spatial distribution and number of IL-33+ cells did not change substantially after infection, IL-33+ cells in LCMV-infected tissue exhibited larger, rounder nuclei, consistent with a more activated status (Figure 2B and data not shown).
Splenic expression of IL-33 is enhanced in FHL2 mice, and expression of ST2 is increased in mice and in patients with FHL2. Spleens and livers from Prf1−/− and WT mice were analyzed before and after LCMV infection. (A) Expression of Il33 at day 0 (Uninf) and at 7 days postinfection (Inf) (n = 4 mice per group). Analyzed by 2-way ANOVA. The number symbol indicates significance of genotype (WT vs Prf1−/−); the double dagger symbol indicates LCMV (Uninf vs Inf); and the asterisk indicates interaction between genotype and LCMV. ##P < .01; ‡‡‡P < .001; *P < .05. (B) Immunohistochemical staining of IL-33 in Prf1−/− mice. Original magnification ×200. Representative of 4 mice per group. (C) IL-33 immunohistochemistry in livers of LCMV-infected Prf1−/− mice 8 days p.i., with or without in vivo administration of ST2-blocking antibody. Representative of 4 mice per group. Original magnification as indicated. (D) Expression of Il1rl1 (ST2 gene), analyzed as in panel A; *P < .05; ‡‡‡P < .001; #P < .05; **P < .01; ##P < .01. (E) Expression of IL1RL1 in peripheral blood mononuclear cells from pediatric patients with FHL2 (n = 3), patients with systemic juvenile idiopathic arthritis (sJIA, n = 18), and healthy control subjects (control 1, n = 33; control 2, n = 29). Data are combined from 2 published data sets (GSE26050 [triangles] and GSE21521 [diamonds]).20,21 Analyzed by 1-way ANOVA; ***P < .001 by Dunnett multiple comparison posttest comparing FHL2 patients to all other groups. ANOVA, analysis of variance; AU, arbitrary units; mRNA, messenger RNA; p.i., postinfection.
Splenic expression of IL-33 is enhanced in FHL2 mice, and expression of ST2 is increased in mice and in patients with FHL2. Spleens and livers from Prf1−/− and WT mice were analyzed before and after LCMV infection. (A) Expression of Il33 at day 0 (Uninf) and at 7 days postinfection (Inf) (n = 4 mice per group). Analyzed by 2-way ANOVA. The number symbol indicates significance of genotype (WT vs Prf1−/−); the double dagger symbol indicates LCMV (Uninf vs Inf); and the asterisk indicates interaction between genotype and LCMV. ##P < .01; ‡‡‡P < .001; *P < .05. (B) Immunohistochemical staining of IL-33 in Prf1−/− mice. Original magnification ×200. Representative of 4 mice per group. (C) IL-33 immunohistochemistry in livers of LCMV-infected Prf1−/− mice 8 days p.i., with or without in vivo administration of ST2-blocking antibody. Representative of 4 mice per group. Original magnification as indicated. (D) Expression of Il1rl1 (ST2 gene), analyzed as in panel A; *P < .05; ‡‡‡P < .001; #P < .05; **P < .01; ##P < .01. (E) Expression of IL1RL1 in peripheral blood mononuclear cells from pediatric patients with FHL2 (n = 3), patients with systemic juvenile idiopathic arthritis (sJIA, n = 18), and healthy control subjects (control 1, n = 33; control 2, n = 29). Data are combined from 2 published data sets (GSE26050 [triangles] and GSE21521 [diamonds]).20,21 Analyzed by 1-way ANOVA; ***P < .001 by Dunnett multiple comparison posttest comparing FHL2 patients to all other groups. ANOVA, analysis of variance; AU, arbitrary units; mRNA, messenger RNA; p.i., postinfection.
Intriguingly, hepatic lymphocytic infiltrates in FHL2 mice uniformly displayed punctate perinuclear IL-33 staining, in contrast to published reports describing IL-33 as a nuclear factor not expressed in lymphocytes (Figure 2C).11,12 An identical IL-33-specific staining pattern was also found in the sparser hepatic inflammatory infiltrates of LCMV-infected WT mice (see supplemental Figure 1, available on the Blood Web site). In vivo blockade of ST2 eliminated this punctate staining without substantially diminishing the number of IL-33+ nuclei, suggesting that IL-33 is internalized by lymphocytes in an ST2-dependent manner (Figure 2C). These data are consistent with receptor-mediated endocytosis of IL-33 and thus raise the possibility that inflammatory lymphocytes in LCMV-infected mice respond to IL-33 via ST2. Accordingly, expression of ST2 (encoded by Il1rl1) was greatly increased in spleens and livers of FHL2 mice compared with LCMV-infected WT mice (Figure 2D). We then examined ST2 expression in peripheral blood mononuclear cells from human FHL2 patients, using public data sets.28,29 Expression of ST2 was highly upregulated in pediatric FHL2 patients compared with healthy children and children with an unrelated inflammatory disease (systemic juvenile idiopathic arthritis) (Figure 2E). Together, these results show that IL-33 and ST2 are highly expressed in mice and humans with FHL2, warranting further investigation of a potential role for the IL-33/ST2 pathway in driving disease.
Blockade of ST2 reduces mortality and morbidity of FHL2 mice
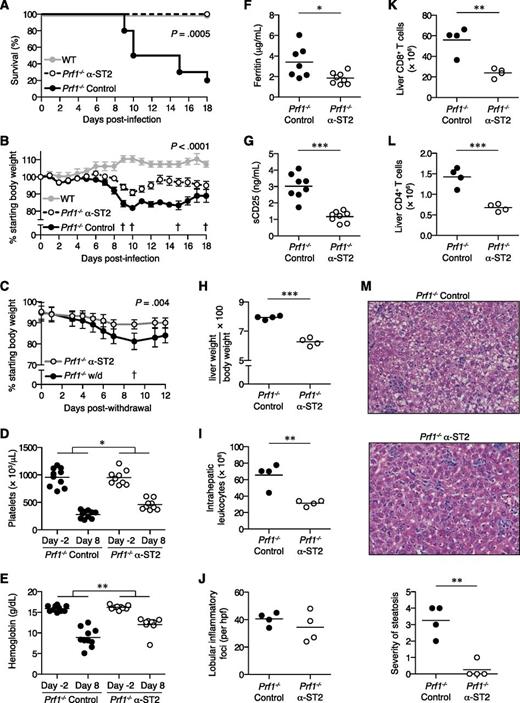
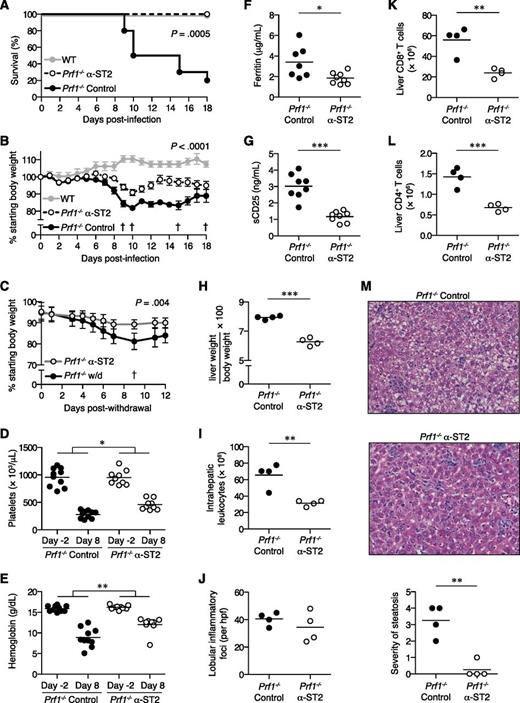
We disrupted IL-33 signaling in FHL2 mice by administration of ST2-blocking antibody (α-ST2). Remarkably, α-ST2-treated mice were significantly protected from mortality and weight loss compared with FHL2 mice receiving isotype control antibody (control mice) (Figures 3A-B). Continual ST2 blockade in α-ST2-treated mice promoted survival ≥30 days postinfection and limited chronic weight loss compared with mice withdrawn from α-ST2 treatment (Figure 3C). In addition, α-ST2-treated mice showed less-severe thrombocytopenia and anemia compared with control mice (Figures 3D-E). Disease activity markers such as ferritin and soluble CD25 were also decreased upon ST2 blockade (Figures 3F-G). Spleen size and cellularity did not differ markedly between control mice and α-ST2-treated mice, with both groups showing expanded white pulp and extramedullary hematopoiesis (supplemental Figure 2 and data not shown). However, ST2 blockade significantly reduced hepatomegaly and total numbers of intrahepatic leukocytes in α-ST2-treated mice compared with control mice, despite similar numbers of lobular inflammatory foci (Figure 3H-J). Numbers of CD8+ and CD4+ T cells were diminished in the livers of α-ST2-treated mice, suggesting a particular dependence on IL-33 signaling by cells known to be hyperactivated in FHL2 mice (Figures 3K-L). Furthermore, liver parenchymal damage in the form of microvesicular steatosis was greatly decreased in α-ST2-treated mice relative to control mice (Figure 3M). Together, these data show that the severity of FHL is significantly reduced by ST2 blockade.
ST2 blockade reduces morbidity and mortality of FHL2 mice.Prf1−/− mice were infected with LCMV to induce FHL2 and treated with either α-ST2 or control antibodies. (A) Survival of α-ST2-treated mice (n = 9) and control mice (n = 10). Representative of 2 independent experiments. Analyzed by log-rank (Mantel-Cox) test. LCMV-infected WT mice (n = 4) are included for visual comparison. (B) Body weight of α-ST2-treated mice and control mice. Symbols represent mean ± standard error of the mean of 9 to 10 mice. The dagger symbol indicates time points at which control mice died and were excluded from subsequent weight analysis. Representative of 2 independent experiments. Analyzed by linear mixed-effects model to allow for missing data due to mouse mortality: treatment and body weight were modeled as fixed effects, and individual mice were treated as a random effect to account for baseline variability between animals (eg, intercept only). Significance of interaction term (α-ST2 vs control over time) is indicated. LCMV-infected WT mice (n = 4) are included for visual comparison. (C) Body weight of Prf1−/− mice withdrawn from α-ST2 treatment and switched to control antibody at day 18 p.i. or receiving continued α-ST2 treatment. Symbols represent mean ± standard error of the mean of 4 to 5 mice. The † symbol indicates the time point at which 1 withdrawal (w/d) mouse died and was excluded from subsequent weight analysis. Analyzed by linear mixed-effects model as in panel B; significance of interaction term (α-ST2 vs w/d over time) is indicated. Platelet counts (D) and hemoglobin levels (E) in peripheral blood of α-ST2-treated Prf1−/− mice (n = 9) and control Prf1−/− mice (n = 10) 2 days prior to infection and 8 days p.i. Representative of 2 independent experiments. Analyzed by repeated-measures 2-way ANOVA; significance of interaction term (α-ST2 vs control over time) is indicated. Ferritin (F) and soluble CD25 (sCD25) levels (G) in serum 8 days p.i. Data pooled from 2 independent experiments, n = 7 to 8 mice per group. (H-M) Liver pathology in α-ST2-treated Prf1−/− mice (n = 4) and control Prf1−/− mice (n = 4) 8 days p.i. Representative of 2 independent experiments. Analyzed by Student 2-tailed t test. (H) Liver weight, expressed as a ratio of total body weight. (I) Total numbers of intrahepatic leukocytes. (J) Numbers of lobular inflammatory foci per ×20 objective high-power field (hpf), enumerated from hematoxylin and eosin (H&E)-stained tissue sections. Total numbers of intrahepatic CD8+ T cells (K) and CD4+ T cells (L), as measured by flow cytometry. (M) Representative H&E-stained liver sections, original magnification ×200. Severity of microvesicular steatosis was assessed on day 8 p.i. using a standardized scoring system as follows: 0, absent; 1, 1% to 20% of area per ×20 objective hpf; 2, 21% to 40%; 3, 41% to 60%; 4, 61% to 80%; 5, 81% to 100%. *P < .05; **P < .01; ***P < .001.
ST2 blockade reduces morbidity and mortality of FHL2 mice.Prf1−/− mice were infected with LCMV to induce FHL2 and treated with either α-ST2 or control antibodies. (A) Survival of α-ST2-treated mice (n = 9) and control mice (n = 10). Representative of 2 independent experiments. Analyzed by log-rank (Mantel-Cox) test. LCMV-infected WT mice (n = 4) are included for visual comparison. (B) Body weight of α-ST2-treated mice and control mice. Symbols represent mean ± standard error of the mean of 9 to 10 mice. The dagger symbol indicates time points at which control mice died and were excluded from subsequent weight analysis. Representative of 2 independent experiments. Analyzed by linear mixed-effects model to allow for missing data due to mouse mortality: treatment and body weight were modeled as fixed effects, and individual mice were treated as a random effect to account for baseline variability between animals (eg, intercept only). Significance of interaction term (α-ST2 vs control over time) is indicated. LCMV-infected WT mice (n = 4) are included for visual comparison. (C) Body weight of Prf1−/− mice withdrawn from α-ST2 treatment and switched to control antibody at day 18 p.i. or receiving continued α-ST2 treatment. Symbols represent mean ± standard error of the mean of 4 to 5 mice. The † symbol indicates the time point at which 1 withdrawal (w/d) mouse died and was excluded from subsequent weight analysis. Analyzed by linear mixed-effects model as in panel B; significance of interaction term (α-ST2 vs w/d over time) is indicated. Platelet counts (D) and hemoglobin levels (E) in peripheral blood of α-ST2-treated Prf1−/− mice (n = 9) and control Prf1−/− mice (n = 10) 2 days prior to infection and 8 days p.i. Representative of 2 independent experiments. Analyzed by repeated-measures 2-way ANOVA; significance of interaction term (α-ST2 vs control over time) is indicated. Ferritin (F) and soluble CD25 (sCD25) levels (G) in serum 8 days p.i. Data pooled from 2 independent experiments, n = 7 to 8 mice per group. (H-M) Liver pathology in α-ST2-treated Prf1−/− mice (n = 4) and control Prf1−/− mice (n = 4) 8 days p.i. Representative of 2 independent experiments. Analyzed by Student 2-tailed t test. (H) Liver weight, expressed as a ratio of total body weight. (I) Total numbers of intrahepatic leukocytes. (J) Numbers of lobular inflammatory foci per ×20 objective high-power field (hpf), enumerated from hematoxylin and eosin (H&E)-stained tissue sections. Total numbers of intrahepatic CD8+ T cells (K) and CD4+ T cells (L), as measured by flow cytometry. (M) Representative H&E-stained liver sections, original magnification ×200. Severity of microvesicular steatosis was assessed on day 8 p.i. using a standardized scoring system as follows: 0, absent; 1, 1% to 20% of area per ×20 objective hpf; 2, 21% to 40%; 3, 41% to 60%; 4, 61% to 80%; 5, 81% to 100%. *P < .05; **P < .01; ***P < .001.
ST2 blockade suppresses the immune response rather than LCMV infection
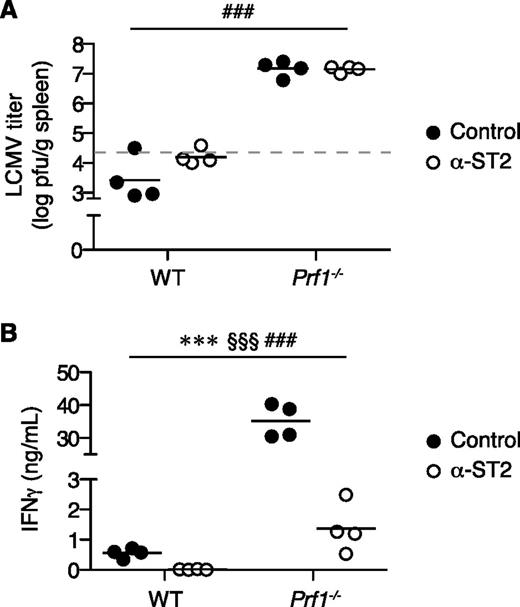
To determine whether the improved outcome of FHL2 mice receiving ST2 blockade was due to enhanced control of viral replication, we measured splenic LCMV titers 8 days postinfection. Despite their ameliorated disease, α-ST2-treated FHL2 mice were no better able to clear LCMV than control FHL2 mice, whereas LCMV-infected WT mice receiving either treatment cleared the infection easily (Figure 4A). These results suggest that rather than modulating resistance to the pathogen, ST2 signaling modifies the pathologic immune response.
ST2 blockade suppresses the immune response rather than LCMV infection. WT and Prf1−/− mice were infected with LCMV, treated with either α-ST2 or control antibodies, and analyzed on day 8 p.i.; n = 4 mice per group. Analyzed by 2-way ANOVA; genotype (WT vs Prf1−/−), ###P < .001; treatment (control vs α-ST2), §§§P < .001; interaction between genotype and treatment, ***P < .001. (A) Splenic LCMV titer. Dotted line indicates limit of detection of plaque assay. Representative of 2 independent experiments. pfu, plaque-forming units. (B) Serum IFNγ level. Representative of 3 independent experiments.
ST2 blockade suppresses the immune response rather than LCMV infection. WT and Prf1−/− mice were infected with LCMV, treated with either α-ST2 or control antibodies, and analyzed on day 8 p.i.; n = 4 mice per group. Analyzed by 2-way ANOVA; genotype (WT vs Prf1−/−), ###P < .001; treatment (control vs α-ST2), §§§P < .001; interaction between genotype and treatment, ***P < .001. (A) Splenic LCMV titer. Dotted line indicates limit of detection of plaque assay. Representative of 2 independent experiments. pfu, plaque-forming units. (B) Serum IFNγ level. Representative of 3 independent experiments.
A critical requirement for immunopathology in FHL is IFNγ, whose serum levels correlate with disease severity.4,30 IL-33 promotes T-cell effector function and synergizes with IL-12 to induce IFNγ production by T cells and natural killer cells26,27,31,32 ; thus, we hypothesized that ST2 blockade reduces disease severity in FHL2 mice by diminishing systemic levels of IFNγ. α-ST2 treatment significantly decreased serum IFNγ in LCMV-infected WT mice but mediated a more striking 16-fold reduction in FHL2 mice (Figure 4B). These data demonstrate that the vast majority of pathogenic IFNγ is under the control of ST2 signaling in FHL.
ST2 blockade decreases numbers of LCMV-specific effector CD8+ and CD4+ T cells in FHL2 mice
To determine how disruption of ST2 reduces systemic IFNγ, we examined the abundance of lymphocytes in α-ST2-treated WT and FHL2 mice. ST2 blockade decreased the number of CD44hiCD62LloCD127lo effector CD8+ T cells in FHL2 mice (2.4-fold reduction), although not to the degree seen in LCMV-infected WT mice (5.4-fold reduction) (Figure 5A-B). Effector CD4+ T cells were similarly reduced in number by α-ST2 treatment (1.7- and 2.2-fold reductions in FHL2 and LCMV-infected WT mice, respectively) (Figure 5C-D). Numbers of CD44hiCD62LhiCD127hi memory-phenotype cells were only slightly altered by α-ST2 treatment in FHL2 mice (1.2-fold reduction in CD8+ T cells) or not at all (1.01-fold increase in CD4+ T cells) (data not shown). Numbers of natural killer cells were unaffected by ST2 blockade in either WT or FHL2 mice (data not shown). Among effector-phenotype T cells, we found lower numbers of LCMV gp33-specific CD8+ T cells in α-ST2-treated FHL2 and LCMV-infected WT mice (Figure 5E-F). Although not statistically significant (P = .054), there was a trend toward a greater α-ST2-mediated reduction of gp33-specific CD8+ T cells in LCMV-infected WT mice (5.9-fold) than in FHL2 mice (2.1-fold) (Figure 5F). Of interest, LCMV gp66-specific CD4+ T cells were reduced 3.5-fold by α-ST2 treatment in FHL2 mice (Figure 5G-H). Together, these findings suggest that ST2 promotes the expansion and/or differentiation of LCMV-specific effector cells, which are known to be pathogenic in FHL2 mice. Furthermore, the suppressive effect mediated by ST2 blockade on effector CD4+ and CD8+ T cells differs in magnitude between LCMV-infected WT and FHL2 mice, highlighting the aberrations in T-cell regulation that are characteristic of FHL.
ST2 blockade decreases numbers of LCMV-specific effector CD8+ and CD4+ T cells in FHL2 mice. WT and Prf1−/− mice were infected with LCMV, treated with either α-ST2 antibodies (open circles) or control antibodies (closed circles), and assessed on day 8 p.i. Analyzed by linear mixed-effects model to account for baseline variability between experimental replicates: treatment and genotype were modeled as fixed effects, and experiment was treated as a random effect (eg, intercept only). The number symbol indicates significance of genotype (WT vs Prf1−/−); the paragraph symbol indicates treatment (control vs α-ST2); and the asterisk indicates interaction between genotype and treatment. Representative flow plots gated on live CD90.2+CD8+ T cells (A) or CD90.2+CD4+ T cells (C), showing effector/memory phenotyping by CD44 and CD62L expression. Numbers of splenic CD44hiCD62LloCD127lo effector CD8+ T cells (B) or CD4+ T cells (D). Data pooled from 4 independent experiments, n = 17 to 18 mice per group. (E) Representative flow plots gated on live CD90.2+CD8+ T cells, showing gp33 major histocompatibility complex (MHC) class I tetramer (tetr) staining. (F) Numbers of splenic gp33-specific CD8+ T cells (n = 13-14 mice per group, data pooled from 3 independent experiments). (G) Representative flow plots gated on live CD90.2+CD4+ T cells, showing gp66 MHC class II tetramer staining. (H) Numbers of splenic gp66-specific CD4+ T cells (n = 9-10 mice per group, data pooled from 2 independent experiments). *P < .05; §§§P < .001; ##P < .01; **P < .01; §§P < .01; ###P < .001.
ST2 blockade decreases numbers of LCMV-specific effector CD8+ and CD4+ T cells in FHL2 mice. WT and Prf1−/− mice were infected with LCMV, treated with either α-ST2 antibodies (open circles) or control antibodies (closed circles), and assessed on day 8 p.i. Analyzed by linear mixed-effects model to account for baseline variability between experimental replicates: treatment and genotype were modeled as fixed effects, and experiment was treated as a random effect (eg, intercept only). The number symbol indicates significance of genotype (WT vs Prf1−/−); the paragraph symbol indicates treatment (control vs α-ST2); and the asterisk indicates interaction between genotype and treatment. Representative flow plots gated on live CD90.2+CD8+ T cells (A) or CD90.2+CD4+ T cells (C), showing effector/memory phenotyping by CD44 and CD62L expression. Numbers of splenic CD44hiCD62LloCD127lo effector CD8+ T cells (B) or CD4+ T cells (D). Data pooled from 4 independent experiments, n = 17 to 18 mice per group. (E) Representative flow plots gated on live CD90.2+CD8+ T cells, showing gp33 major histocompatibility complex (MHC) class I tetramer (tetr) staining. (F) Numbers of splenic gp33-specific CD8+ T cells (n = 13-14 mice per group, data pooled from 3 independent experiments). (G) Representative flow plots gated on live CD90.2+CD4+ T cells, showing gp66 MHC class II tetramer staining. (H) Numbers of splenic gp66-specific CD4+ T cells (n = 9-10 mice per group, data pooled from 2 independent experiments). *P < .05; §§§P < .001; ##P < .01; **P < .01; §§P < .01; ###P < .001.
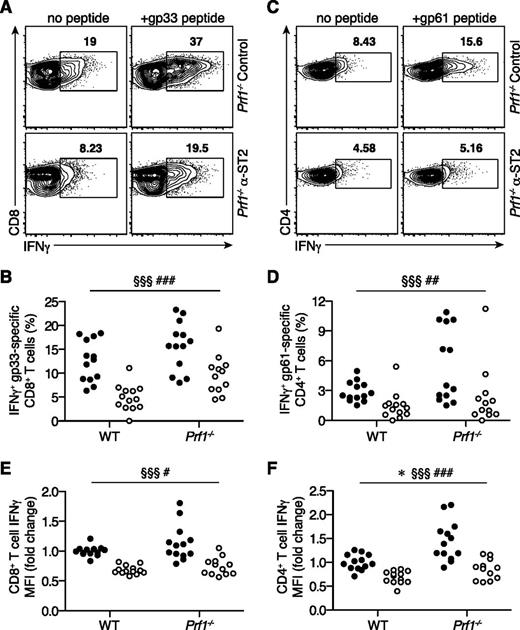
The frequency and IFNγ production capacity of IFNγ+ LCMV-specific T cells are reduced in FHL2 mice receiving ST2 blockade
We next investigated whether ST2 affects the ability of LCMV-specific effector cells to produce IFNγ. Control FHL2 mice demonstrated higher frequencies of IFNγ+ gp33-specific CD8+ T cells than control LCMV-infected WT mice (Figure 6A-B), consistent with previous studies.4,5 Remarkably, in vivo ST2 blockade reduced the frequency of IFNγ+ gp33-specific CD8+ T cells in FHL2 and LCMV-infected WT mice to a similar degree (1.7- and 2.7-fold reductions, respectively) (Figure 6B). Likewise, for CD4+ T cells, α-ST2 treatment decreased the frequency of IFNγ+ gp61-specific CD4+ T cells (2.7- and 2.2-fold reductions in FHL2 and LCMV-infected WT mice, respectively) (Figure 6C-D). In addition, α-ST2 treatment reduced IFNγ median fluorescence intensity in IFNγ+ LCMV-specific CD8+ and CD4+ T cells (Figure 6E-F), suggesting lower transcription or translation of this cytokine. Blockade of ST2 signaling therefore decreases both the frequency and average per-cell IFNγ production of IFNγ+ LCMV-specific T cells to cumulatively lower systemic IFNγ in FHL2 mice.
The frequency and IFNγ production capacity of IFNγ+ LCMV-specific T cells are reduced in FHL2 mice receiving ST2 blockade. WT and Prf1−/− mice were infected with LCMV, treated with either α-ST2 antibodies (open circles) or control antibodies (closed circles), and assessed on day 8 p.i. (n = 12-13 mice per group, data pooled from 3 independent experiments). Analyzed by linear mixed-effects model to account for baseline variability between experimental replicates: treatment and genotype were modeled as fixed effects, and experiment was treated as a random effect. The number symbol indicates significance of genotype (WT vs Prf1−/−); the paragraph symbol indicates treatment (control vs α-ST2); and the asterisk indicates interaction between genotype and treatment. Representative flow plots gated on live CD90.2+CD8+ T cells (A) or CD90.2+CD4+ T cells (C), showing intracellular IFNγ expression in response to in vitro gp33 (A) or gp61 (C) peptide stimulation. Summary data showing frequencies of IFNγ+ LCMV-specific CD8+ T cells (B) and CD4+ T cells (D). Summary data showing median IFNγ fluorescence intensity (MFI) of CD8+ T cells (E) and CD4+ T cells (F) producing IFNγ in response to restimulation with LCMV peptides. MFI is normalized to WT control mean for each experiment. §§§P < .001; ###P < .001; ##P < .01; #P < .05; *P < .05.
The frequency and IFNγ production capacity of IFNγ+ LCMV-specific T cells are reduced in FHL2 mice receiving ST2 blockade. WT and Prf1−/− mice were infected with LCMV, treated with either α-ST2 antibodies (open circles) or control antibodies (closed circles), and assessed on day 8 p.i. (n = 12-13 mice per group, data pooled from 3 independent experiments). Analyzed by linear mixed-effects model to account for baseline variability between experimental replicates: treatment and genotype were modeled as fixed effects, and experiment was treated as a random effect. The number symbol indicates significance of genotype (WT vs Prf1−/−); the paragraph symbol indicates treatment (control vs α-ST2); and the asterisk indicates interaction between genotype and treatment. Representative flow plots gated on live CD90.2+CD8+ T cells (A) or CD90.2+CD4+ T cells (C), showing intracellular IFNγ expression in response to in vitro gp33 (A) or gp61 (C) peptide stimulation. Summary data showing frequencies of IFNγ+ LCMV-specific CD8+ T cells (B) and CD4+ T cells (D). Summary data showing median IFNγ fluorescence intensity (MFI) of CD8+ T cells (E) and CD4+ T cells (F) producing IFNγ in response to restimulation with LCMV peptides. MFI is normalized to WT control mean for each experiment. §§§P < .001; ###P < .001; ##P < .01; #P < .05; *P < .05.
Discussion
This study reveals a previously undescribed role for ST2 in FHL pathophysiology. Expression of both IL-33 and ST2 is increased in tissues of FHL2 mice. Danger signals provided by IL-33 enhance morbidity and mortality in murine FHL2 by intensifying aberrant immune responses, independent of viral load. Notably, ST2 signaling promotes LCMV-specific CD8+ and CD4+ T-cell activation and production of IFNγ, leading to systemic accumulation of supraphysiological IFNγ levels and subsequent lethal inflammation.
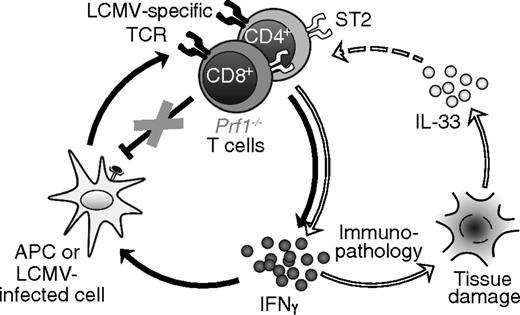
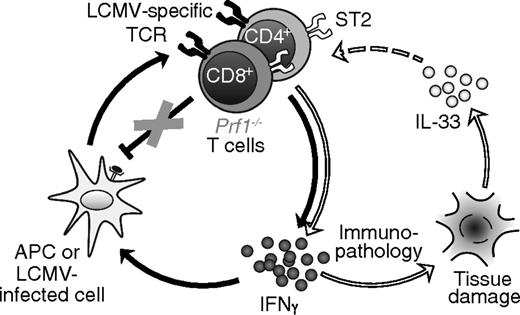
Our data demonstrate that adjuvant-like TCR-independent signals critically contribute to the hyperinflammation of FHL and suggest a revised model for this disease, in which IL-33, likely released from damaged tissue, couples with excessive antigen to drive T cell–mediated production of IFNγ and thereby FHL immunopathology (Figure 7). This model of a 2-signal requirement (ie, persistent antigen and specific danger signals) addresses several key aspects of FHL not previously accounted for: it explains why antigen alone is insufficient to induce FHL in genetically susceptible individuals, and provides a reason for the requirement of MyD88 in the development of FHL.
Proposed model of FHL pathophysiology. In the traditional paradigm (left circle, connected by black arrows), antigen-presenting cells (APCs) and LCMV-infected cells present viral antigens to LCMV-specific T cells, activating them to secrete IFNγ. IFNγ acts back on APCs to enhance their ability to present antigen, setting up a positive feedback loop. In Prf1−/− mice, T cells are unable to eliminate APCs via perforin, resulting in a loss of negative regulation that enables feed-forward amplification of inflammation. In our revised model, IL-33 signaling (right circle, connected by white arrows) further amplifies this vicious cycle. Tissue damage and cell death leads to the release of IL-33, which acts either directly or indirectly on LCMV-specific T cells to further promote their production of IFNγ. Elevated IFNγ exacerbates immunopathology, leading to further release of IL-33 from dying cells.
Proposed model of FHL pathophysiology. In the traditional paradigm (left circle, connected by black arrows), antigen-presenting cells (APCs) and LCMV-infected cells present viral antigens to LCMV-specific T cells, activating them to secrete IFNγ. IFNγ acts back on APCs to enhance their ability to present antigen, setting up a positive feedback loop. In Prf1−/− mice, T cells are unable to eliminate APCs via perforin, resulting in a loss of negative regulation that enables feed-forward amplification of inflammation. In our revised model, IL-33 signaling (right circle, connected by white arrows) further amplifies this vicious cycle. Tissue damage and cell death leads to the release of IL-33, which acts either directly or indirectly on LCMV-specific T cells to further promote their production of IFNγ. Elevated IFNγ exacerbates immunopathology, leading to further release of IL-33 from dying cells.
Disruption of either MyD88 or ST2 signaling in FHL2 mice dramatically ameliorates disease, with reductions in cytopenias, hepatitis, serum IFNγ, and LCMV-specific CD8+ T cells. Because ST2 is upstream of MyD88, these results suggest that many of the improvements effected by MyD88 deficiency in this model are due to loss of IL-33 signaling. However, our data also show that disruption of ST2 does not phenocopy MyD88 deficiency in FHL2 mice, because certain disease markers such as splenomegaly and hepatic lobular inflammation are unchanged by ST2 blockade. Thus, although IL-33 is a major MyD88-dependent inflammatory amplifier, it is possible that additional signaling mediators upstream of MyD88, such as other IL-1 family receptors and TLRs, also contribute. For example, neutralization of IL-18 mitigates organ damage in FHL2 mice despite its inability to reduce mortality.4,33 Deficiency of IL-1R, TLR7, or TLR9 fails to restrict expansion of LCMV-specific CD8+ T cells,9,34 but a role for these pathways in combination with more dominant signals such as ST2 has not been investigated.
Although our data clearly demonstrate an important role for IL-33/ST2 in FHL pathophysiology, several questions remain. The precise triggers resulting in release of IL-33 in murine FHL2 are unknown. In WT mice, splenic IL-33 expression increases upon LCMV infection (see Bonilla et al26 and Figure 2A). Despite the noncytopathic nature of this virus,35 extracellular IL-33 is present in sufficient quantities to robustly activate CD8+ T cells in WT mice, as well as in FHL2 mice.26 Thus, although direct viral effects may not be responsible for such IL-33 release, there is evidently another mechanism of cell injury (likely mediated by the antiviral immune response) that accounts for escape of bioactive IL-33 in both WT and FHL2 mice. Given the large degree of tissue damage observed in FHL2 mice,4 it remains possible that other insults, such as macrophage-derived reactive oxygen species, also promote IL-33 release. Recent reports have highlighted the ability of nonlethal cellular stressors to induce secretion of IL-33,10,36 an additional mechanism that may account for IL-33 release in FHL2 mice.
Our findings reveal an ST2 signaling requirement of CD8+ and CD4+ T cells for IFNγ overproduction in FHL2 mice, but it is unclear at present whether this requirement is T-cell intrinsic or extrinsic. WT CD8+ and CD4+ T cells upregulate ST2 after LCMV infection, and their differentiation and function is regulated by IL-33 in a cell-intrinsic manner26,27 ; furthermore, CD8+ T cells intrinsically require the ST2 signaling adaptor MyD88 for optimal accumulation of LCMV-specific effector cells after infection.37,38 However, the data presented here demonstrate differential effects of ST2 blockade in WT and FHL2 mice, making it difficult to extrapolate directly from previous studies. A wide range of immune cell subsets expresses ST2,14 raising the possibility of an intermediate IL-33-responsive cell in FHL2 pathophysiology. It is also conceivable that α-ST2 treatment modulates IL-33 signaling by blocking soluble ST2; however, because the known biological effects of IL-33 on T cells are decreased in α-ST2-treated mice, it is unlikely that removal of this decoy receptor, leading to elevated free IL-33, would account for our findings. The key pathways linking IL-33 and T cell–derived IFNγ, as well as the long-term effect of ST2 blockade on T-cell function, are the subject of ongoing investigation.
This pathogenic IL-33/IFNγ axis may provide an important point of manipulation for the treatment of FHL, particularly because ST2 expression is elevated in peripheral blood mononuclear cells from FHL2 patients (Figure 2E). Whereas numerous cytokines have been experimentally modulated in FHL mice, IFNγ and, now, IL-33/ST2 are the only immune mediators whose blockade demonstrates therapeutic benefit.4,9 Disruption of ST2 signaling reduces systemic IFNγ to sublethal levels without rendering FHL2 mice completely deficient in this key antiviral cytokine; accordingly, viral control is not worsened in these mice (Figure 5). Targeting IL-33/ST2 is therefore potentially safer than targeting IFNγ directly, because patients with disrupted IFNγ signaling show increased susceptibility to infection, and genetic deletion of the IFNγ receptor in FHL3 mice enhances mortality.9,39,40 Furthermore, recently described cases of FHL occurring in patients with IFNγ-receptor deficiency highlight the need for therapeutics designed to target a diverse range of pathways in FHL.41 Our study demonstrates that ST2 blockade is protective in FHL2 mice after infection but before the development of overt disease. Thus, it is unclear whether the optimal use of ST2 blockade would be for treatment of flare or for prophylaxis, and any future study in human disease might need to consider both possibilities.
Given the decisive role that IL-33/ST2 plays in FHL2, it is interesting to speculate that IL-33 may also drive inflammation in other forms of hemophagocytic syndrome. Elevated IFNγ and CD8+ T cells are key features of all murine models of FHL and several models of acquired hemophagocytic lymphohistiocytosis,2 suggesting the possibility of a common mechanism of IL-33 driving T-cell production of IFNγ in these disorders. Furthermore, IL-33 may drive inflammation in cytokine storm disorders more generally, because IL-33 has already been implicated in endotoxic shock42 and hantavirus infection.43 It will be important to determine whether α-ST2 therapy could benefit a broader range of immune-mediated diseases than the disorders of type 2 immunity with which IL-33 is classically identified.
In summary, we have demonstrated that disruption of ST2 signaling in the murine model of FHL reduces T cell–mediated production of IFNγ, leading to improved morbidity and mortality, and suggest blockade of this pathway as a viable treatment strategy for FHL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joanne Mauger and Jacquelyn Freund for technical assistance; Dirk Smith for providing α-ST2 and control antibodies; and the laboratories of Taku Kambayashi, Martha Jordan, Paula Oliver, and Hamid Bassiri for thoughtful input.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grant R01 HL112836-A1 (E.M.B.) and National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 5T32AR007442-27 (J.E.R.), and by the Nancy Taylor Foundation (E.M.B.).
Authorship
Contribution: J.E.R. and S.R. designed, conducted, and analyzed the experiments; N.C. and E.S. assisted in conducting the experiments; M.P. and P.A.K. conducted and analyzed the histologic studies; E.S. and E.J.W. provided tools and valuable discussion; J.E.R. prepared the manuscript figures and wrote the manuscript; J.E.R. and E.M.B. edited the manuscript; and E.M.B. supervised the overall research.
Conflict-of-interest disclosure: J.E.R. and E.M.B. are named on US Provisional Patent No. 61/982, 026 “Compositions and Methods for Treating Hemophagocytic Lymphohistiocytosis” for treatment of FHL with IL-33 blockade. The remaining authors declare no competing financial interests.
Correspondence: Edward M. Behrens, The Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, 1102 Abramson Research Center, Philadelphia, PA 19104; e-mail: behrens@email.chop.edu.

![Figure 2. Splenic expression of IL-33 is enhanced in FHL2 mice, and expression of ST2 is increased in mice and in patients with FHL2. Spleens and livers from Prf1−/− and WT mice were analyzed before and after LCMV infection. (A) Expression of Il33 at day 0 (Uninf) and at 7 days postinfection (Inf) (n = 4 mice per group). Analyzed by 2-way ANOVA. The number symbol indicates significance of genotype (WT vs Prf1−/−); the double dagger symbol indicates LCMV (Uninf vs Inf); and the asterisk indicates interaction between genotype and LCMV. ##P < .01; ‡‡‡P < .001; *P < .05. (B) Immunohistochemical staining of IL-33 in Prf1−/− mice. Original magnification ×200. Representative of 4 mice per group. (C) IL-33 immunohistochemistry in livers of LCMV-infected Prf1−/− mice 8 days p.i., with or without in vivo administration of ST2-blocking antibody. Representative of 4 mice per group. Original magnification as indicated. (D) Expression of Il1rl1 (ST2 gene), analyzed as in panel A; *P < .05; ‡‡‡P < .001; #P < .05; **P < .01; ##P < .01. (E) Expression of IL1RL1 in peripheral blood mononuclear cells from pediatric patients with FHL2 (n = 3), patients with systemic juvenile idiopathic arthritis (sJIA, n = 18), and healthy control subjects (control 1, n = 33; control 2, n = 29). Data are combined from 2 published data sets (GSE26050 [triangles] and GSE21521 [diamonds]).20,21 Analyzed by 1-way ANOVA; ***P < .001 by Dunnett multiple comparison posttest comparing FHL2 patients to all other groups. ANOVA, analysis of variance; AU, arbitrary units; mRNA, messenger RNA; p.i., postinfection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/4/10.1182_blood-2015-07-659813/4/m_426f2.jpeg?Expires=1765928266&Signature=1mcbgFUxneI0Kroh~ks1jYxAK-YqnjiSA5aXY2EwP~93CbRsH9wrjKo9JOMBtHZ1fJkJ0MAdmb7lj0rxoVjP75B8u6BdLc~uvzBeGvPG0q09TMiO~VY65drJscqD2oetBNBinQ9UIZC02wrNk3Jzy7HXZ9uwN-kN43NcgA3oRPAegRQ2x3hNCB~FRpZcaGa6JK-Y922VPgbsSian1x2efg16umnq8YwiFXgSDWV6nT7-Yrs5unWTTIyoMkHIyaB5RlqqgzSEgOYW10lORephlNsBLo~29-ZlFmslzmzXkWEF5K8UtGEv9O0FLqKYzQBFAInXfwZ6M-JI135pCnurIQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 2. Splenic expression of IL-33 is enhanced in FHL2 mice, and expression of ST2 is increased in mice and in patients with FHL2. Spleens and livers from Prf1−/− and WT mice were analyzed before and after LCMV infection. (A) Expression of Il33 at day 0 (Uninf) and at 7 days postinfection (Inf) (n = 4 mice per group). Analyzed by 2-way ANOVA. The number symbol indicates significance of genotype (WT vs Prf1−/−); the double dagger symbol indicates LCMV (Uninf vs Inf); and the asterisk indicates interaction between genotype and LCMV. ##P < .01; ‡‡‡P < .001; *P < .05. (B) Immunohistochemical staining of IL-33 in Prf1−/− mice. Original magnification ×200. Representative of 4 mice per group. (C) IL-33 immunohistochemistry in livers of LCMV-infected Prf1−/− mice 8 days p.i., with or without in vivo administration of ST2-blocking antibody. Representative of 4 mice per group. Original magnification as indicated. (D) Expression of Il1rl1 (ST2 gene), analyzed as in panel A; *P < .05; ‡‡‡P < .001; #P < .05; **P < .01; ##P < .01. (E) Expression of IL1RL1 in peripheral blood mononuclear cells from pediatric patients with FHL2 (n = 3), patients with systemic juvenile idiopathic arthritis (sJIA, n = 18), and healthy control subjects (control 1, n = 33; control 2, n = 29). Data are combined from 2 published data sets (GSE26050 [triangles] and GSE21521 [diamonds]).20,21 Analyzed by 1-way ANOVA; ***P < .001 by Dunnett multiple comparison posttest comparing FHL2 patients to all other groups. ANOVA, analysis of variance; AU, arbitrary units; mRNA, messenger RNA; p.i., postinfection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/4/10.1182_blood-2015-07-659813/4/m_426f2.jpeg?Expires=1766014710&Signature=f3ObY4-Ro~sLmyGcBTVuCGOk97JfByRohqHTirMfxLdGS39RVPFxCjeRvSC5HE0~m7WfVWsD8gGQriMCy~XSGcVfCg6l6SvKgtcqNOMtC-4qCZDBPatpCW7tZleDJvzxWUjwPPbUECPpr8bv7WcC9b7Bp5QLYpP9bJBLL6YMzIvoh3LfCIZ-Xr3QgDtDTKqxu3d6kUhZ4mrcFttKGw9kTV62nBr~24zXbFgAYEwfVy8dfPc76NZtCNMS9stFHTDvxKEXKp8fltmaWvQf1kSzRHOiFdHxAHLpzHx9q9W7st1iqDK~BpjLo04zPyHncQPlbEKwj~8-mTs62gzmfBZ2AQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)