In this issue of Blood, Joshi et al demonstrate that the interaction between fibrin and αMβ2 on leukocytes reduces the development of liver fibrosis in a mouse model of cholestatic injury.1
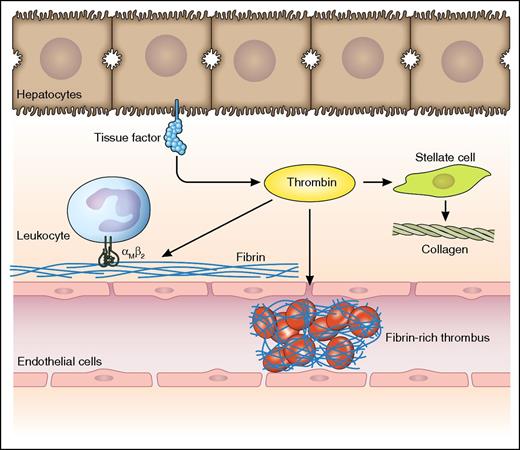
Potential consequences of intrahepatic activation of coagulation following liver injury. Liver injury results in decryption of hepatocyte (and/or biliary epithelial cell) tissue factor which results in the generation of thrombin. Thrombin potentially activates both pro- and antifibrogenic pathways: (1) It generates fibrin, which in an αMβ2-dependent manner binds leukocytes resulting in antifibrogenic effects.1 (2) It activates stellate cells resulting in enhanced collagen production.6 (3) It generates fibrin in the sinusoidal space resulting in microischemia and progression of disease.7 Professional illustration by Patrick Lane, ScEYEnce Studios.
Potential consequences of intrahepatic activation of coagulation following liver injury. Liver injury results in decryption of hepatocyte (and/or biliary epithelial cell) tissue factor which results in the generation of thrombin. Thrombin potentially activates both pro- and antifibrogenic pathways: (1) It generates fibrin, which in an αMβ2-dependent manner binds leukocytes resulting in antifibrogenic effects.1 (2) It activates stellate cells resulting in enhanced collagen production.6 (3) It generates fibrin in the sinusoidal space resulting in microischemia and progression of disease.7 Professional illustration by Patrick Lane, ScEYEnce Studios.
Importantly, administration of leukadherin-1, a small molecule that enhances the fibrin-αMβ2 interaction, reduces already established fibrosis, suggesting that this interaction is a promising therapeutic target.
Cholestatic liver diseases are a significant clinical challenge. They may adversely affect the quality of life due to pruritis and fatigue, for which limited treatment options are available. In addition, patients may develop cirrhosis with symptoms of portal hypertension, hepatic encephalopathy, and eventually liver failure. Despite advances in medical management, liver transplant is the only therapeutic option for patients with end-stage disease. Therapeutic interventions that would slow down disease progression would, therefore, be of interest.
There are, unfortunately, significant knowledge gaps that need to be filled before a fibrin-directed therapeutic intervention is ready for clinical trial. First, the fibrin-αMβ2 interaction reduces fibrosis in a model of cholestatic liver disease, but does not appear to affect fibrosis development in a model of noncholestatic disease. This selectivity may be explained by the observation that the fibrin-αMβ2 interaction reduces bile duct hyperplasia, a phenomenon exclusively observed in cholestatic liver disease. The authors suggest that dampening of fibrogenesis by the fibrin-αMβ2 interaction occurs due to the reduction of bile duct hyperplasia and provide evidence that this is from suppression of proinflammatory signals. Further study will be required to confirm that the fibrin-αMβ2 interaction indeed exclusively reduces cholestatic fibrosis. In addition, it is at present unclear whether the selective reduction of biliary fibrosis translates to true clinical improvement, as no differences in liver injury or liver function markers upon modulation of the fibrin-αMβ2 interaction were demonstrated in this model. Second, although intrahepatic deposition of fibrin is central to the protective effect of the fibrin-αMβ2 interaction in cholestatic disease, multiple undesirable effects of intrahepatic generation of fibrin have been identified. Intrahepatic deposition of fibrin accompanies cholestatic and noncholestatic chronic liver injury in mice.1 It has been demonstrated that hepatocytes2 and bile duct epithelial cells3 contain tissue factor, which is in physiology in a noncoagulant (ie, encrypted4 ) state. Upon liver injury, tissue factor can be decrypted, and become able to activate coagulation. Liver-derived tissue factor likely drives intrahepatic activation of coagulation with subsequent fibrin deposition, as was recently demonstrated in a mouse model of cholestasis by bile duct ligation.5 Intrahepatic activation of coagulation results in fibrin deposition, which potentially not only sets the repair mechanism identified by Joshi and coworkers into motion, but may also activate damaging mechanisms. It has been demonstrated that thrombin activates hepatic stellate cells, which are the collagen-producing cells central in liver fibrogenesis.6 In addition, it has been suggested that intrahepatic generation of microthrombi contribute to progression of fibrosis by causing local tissue ischemia.7 A randomized controlled trial has demonstrated delayed disease progression by long-term administration of low-molecular-weight heparin in patients with noncholestatic cirrhosis.8 Such therapeutic strategies likely decrease intrahepatic deposition of fibrin. It will be of interest to study the effect of long-term anticoagulant therapy in patients with cholestatic disease. Theoretically, patients with cholestatic disease, who are more hypercoagulable,9 might benefit more from anticoagulants than patients with noncholestatic disease. On the other hand, anticoagulants may interfere with the protective effect of the fibrin-αMβ2 interaction and result in accelerated disease progression in patients with cholestatic disease.
It is unclear why the protective effects of fibrinogen overrule the potentially harmful effects of intrahepatic activation of coagulation in this mouse model of cholestatic liver disease used by the authors. It cannot be excluded that the net effect of intrahepatic fibrin formation on disease progression depends on the experimental model and the timing or mode of intervention. Notably, using an acute bile duct injury model, these authors have demonstrated fibrin deposition to have detrimental effects, supporting the theory that the effect of intrahepatic fibrin deposition is strongly context-dependent.10 The potential pro- and antifibrogenic properties of intrahepatic fibrin deposition in livers with cholestatic disease are shown in the figure. These opposing drivers should be taken into account in the future design of therapeutic intervention strategies.
Despite these potential caveats, the demonstration by Joshi and coworkers of successful treatment of established cholestatic fibrosis by a small molecule that enhances the fibrin-αMβ2 interaction is a major achievement. In addition, the authors have demonstrated a pivotal role of factor XIII (FXIII) in protecting the liver against progression of cholestatic injury. As plasma levels of FXIII decrease in advancing liver disease, supplementation of FXIII concentrate may be yet another antifibrotic therapeutic strategy to emerge from this study.
Conflict-of-interest disclosure: The author declares no competing financial interests.