In this issue of Blood, Barr et al report the results of the combination of idelalisib and entospletinib, discussing why “we” need to carefully design clinical trials with novel agents with an increased focus on safety in addition to thoroughly incorporating biomarkers to predict unique toxicities.1
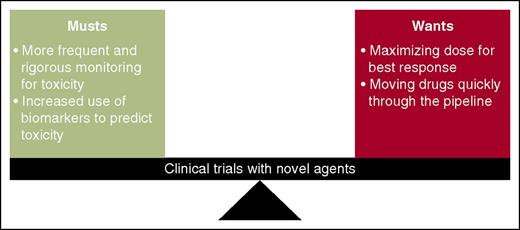
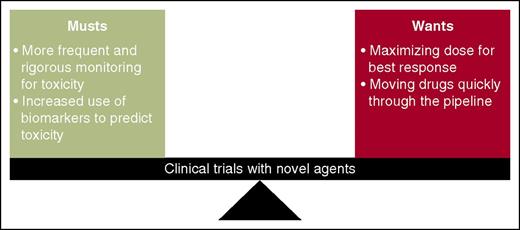
A harmony between “musts” and “wants” in clinical trials is essential to ultimately increase survival. Barr et al use idelalisib and entospletinib to illustrate the necessity of more rigorous toxicity management and increased use of pharmacodynamics/biomarker monitoring in early-phase clinical trials. This must be balanced with our desire to maximize dose for best response and to quickly move drugs to later-phase clinical trials.
A harmony between “musts” and “wants” in clinical trials is essential to ultimately increase survival. Barr et al use idelalisib and entospletinib to illustrate the necessity of more rigorous toxicity management and increased use of pharmacodynamics/biomarker monitoring in early-phase clinical trials. This must be balanced with our desire to maximize dose for best response and to quickly move drugs to later-phase clinical trials.
B-cell receptor (BCR) signaling is critical for the growth of B lymphocytes and plays a large role in the development of chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphomas (NHLs). In contrast to chemoimmunotherapy programs, agents targeting molecules downstream of the BCR pathway, such as Bruton tyrosine kinase, phosphatidylinositol 3-kinase (PI3K), and spleen tyrosine kinase (SYK), are revolutionizing the way we manage these patients—especially those that are older, unfit, and/or at high risk. Although these new agents show remarkable efficacy as monotherapies, the time to complete remission and presence of minimal residual disease (MRD) have led to developing combination trials with the goals of increasing the depth of response, decreasing the time to response, and eliminating MRD.
In this study, Barr et al report the results of 2 novel agents, idelalisib (a PI3Kδ inhibitor) and entospletinib (a SYK inhibitor) in patients with relapsed/refractory CLL and NHL, based on data of synergistic inhibition of CLL cells with the combination in vitro.2 Five cohorts of 66 patients underwent rapid intrapatient dose escalation every 2 or 4 weeks, with responses assessed every 8 weeks, and cytokine/chemokine levels measured at 1, 2, 3, 4, 6, 8, 12, 16, and 20 weeks. Although noting activity with this combination—overall response rates (ORRs) of 60% for patients with CLL and 36% for patients with follicular lymphoma—the study was terminated early because of treatment-related pneumonitis. This occurred in 12 patients, including 11 patients with grade ≥3 pneumonitis, of which 2 died. A significant increase in cytokines/chemokines associated with immune-cell recruitment were observed in the blood of patients who developed pneumonitis starting around week 4 and were most pronounced by week 12, long after the rapid dose escalations.
This article highlights not only the efficacy of these novel agents, but also the increased toxicity that requires a heightened awareness by investigators given the unique mechanisms of action of these drugs compared with more traditional chemoimmunotherapy programs. As we integrate these novel agents into our treatment paradigms for CLL and NHL, either in combination with other novel agents or with chemotherapy, we need to incorporate more rigorous biomarker monitoring to predict potentially deadly toxicities before a dose escalation is made. This is not only to protect our patients, but to also prevent the “death” of a potentially active agent in development that could have been avoided with a more conservative trial design.
This is not the first time we have seen this with the development of novel agent(s) in CLL and NHL. Lenalidomide was initially evaluated in relapsed/refractory CLL given its efficacy in patients with del(5q) myelodysplastic syndrome and multiple myeloma. In these diseases and at doses of 10 to 25 mg, toxicities such as tumor flare reaction (TFR) and tumor lysis syndrome (TLS) were not seen. The first phase 2 study evaluated lenalidomide in relapsed/refractory CLL at 25 mg on days 1 through 21 every 28 days, whereas the second trial evaluated 10 mg daily with rapid dose escalation to 25 mg. Although there was activity, ORRs were 47% and 38%, respectively; significant TFR and TLS were noted.3,4 Celgene summarized the 240 CLL patients who were being treated with lenalidomide at that time; TLS was observed in 7 patients, including 2 deaths, and TFR was present in the majority of patients to varying degrees. In retrospect, bulky disease, renal insufficiency, and elevated uric acid were associated with development of TLS.5 These findings led to the temporary suspension of the study of lenalidomide in CLL for some time. Later trials used a more careful dose escalation schedule, used biomarkers such as uric acid level and lymph node size with increased monitoring for TLS and TFR, and instituted prophylaxis with intravenous fluids and allopurinol, which resulted in marked improvement of the safety profile. Despite this, there has been a decline in its clinical use in CLL.
We also recently witnessed this phenomenon with the development of ABT-199 (venetoclax), a first-in-class BCL-2 inhibitor, in relapsed/refractory CLL and NHL.6 In the phase 1 dose escalation study in CLL, patients received a single dose of ABT-199 on day −3 or −7 before week 1 and then received a once-daily continuous dose. Doses ranged from 50 mg to 1200 mg. Remarkable results were seen, with an ORR of 84%, but 7 treatment-emergent dose-limiting toxicities arose, in which 5 were from TLS and 1 was from a sudden death in the setting of severe TLS. Similar to lenalidomide, the study of ABT-199 in relapsed/refractory CLL was temporarily suspended. Protocol modifications were made and patients are now classified by risk of developing TLS via factors such as absolute lymphocyte count and lymph node size with a conservative slow “ramp-up” dose escalation.7
The arrival of these and other novel agents and designing rational combinations present a challenge for investigators. Although they provide hope for our heavily pretreated, unfit, and high-risk patients given their activity, unique mechanisms of action carry unique mechanisms of toxicity. We are discovering drugs that may yield unprecedented rates of traditional toxicities, such as TLS, and for those agents that harness the power of the immune system, an entirely new class of toxicities such as pneumonitis. It is time to completely shed the “1-size-fits-all” mentality with drug development and more rigorously incorporate biomarker, pharmacogenetic, and pharmacogenomic testing into all clinical trials, not only to predict who will respond best to a drug or combination, but also to identify who is likely to be at greatest risk of toxicity (see figure). By continuing to focus our efforts on the underlying disease biology, mechanisms of action of these agents, and paying careful attention to patient safety in the design of future trials, we can reduce toxicities that may limit a drug’s development and fulfill that promise we all made in medical school: “I will do no harm.”8
Conflict-of-interest disclosure: N.L. receives research support from Gilead, Genentech, Infinity, Pronai, and Abbvie; is on the advisory board for Gilead, Genentech, Pharmacyclics, and Abbvie; and is also a committee member for Celgene. S.H.B. declares no competing financial interests.