Key Points
CD4+ T-cell responses in 2 patients with acquired TTP.
CUB2 domain-derived core peptides are recognized by CD4+ T cells present in 2 patients with acquired TTP.
Abstract
Acquired thrombotic thrombocytopenic purpura (TTP) is a life-threatening disorder resulting from the development of autoantibodies against ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). HLA-DRB1*11 provides a risk factor for developing acquired TTP. Pulsing of antigen-presenting cells from HLA-DRB1*11– and HLA-DRB1*03–positive individuals with ADAMTS13 resulted in presentation of peptides derived from the CUB2 domain of ADAMTS13 with core sequences FINVAPHAR or ASYILIRD. Here, we assessed whether FINVAPHAR- or ASYILIRD-reactive CD4+ T cells are present in peripheral blood mononuclear cells from HLA-DRB1*11 and HLA-DRB1*03–positive subjects with acquired TTP. The presence of ADAMTS13-reactive CD4+ T cells was addressed by flow cytometry and the expression of activation marker CD40 ligand by CD4+ T cells. FINVAPHAR-reactive CD4+ T cells were identified in an HLA-DRB1*11–positive patient during the acute phase of the disease whereas ASYILIRD-positive CD4+ T cells were identified in a DRB1*03-positive patient with acquired TTP. Frequencies of CUB2 domain-reactive CD4+ T cells ranged from 3.3% to 4.5%. Control peptides in which the anchor residues were modified did not induce activation of CD4+ T cells. Taken together, our data provide evidence for the involvement of CUB2 domain-reactive CD4+ T cells in the etiology of acquired TTP.
Introduction
The autoimmune disorder thrombotic thrombocytopenic purpura (TTP) is characterized by the development of autoantibodies against ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) in previously healthy individuals.1-4 Most patients affected by TTP present antibodies of the immunoglobulin class G, predominantly directed toward an exposed loop in the spacer domain.2,5-7 The production of high-affinity antibodies of subclass immunoglobulin class G requires the help of CD4+ T cells.8 Recently, HLA-DRB1*11 has been reported to be a risk factor for the development of acquired TTP.9-11 We have explored the repertoire of ADAMTS13 peptides presented on major histocompatibility complex class II (MHC-II).12 Pulsing of dendritic cells from healthy donors showed preferential presentation of the CUB2 domain-derived peptide FINVAPHAR on HLA-DRB1*11.12 In addition, we observed presentation of a CUB2 domain peptide with the core sequence ASYILIRD on antigen-presenting cells derived from HLA-DRB1*03–positive individuals.12 In this study, we assessed whether patients affected by TTP carry CD4+ T cells reactive toward these CUB2 domain-derived peptides. Antigen-specific CD4+ T cells were identified using the activation marker CD40 ligand (CD40L; CD154).13-15 CD40L expression is induced following binding of the T-cell receptor (TCR) to antigenic peptides presented on MHC-II.16 In this study, we screened peripheral blood mononuclear cells (PBMCs) from HLA-typed healthy donors and patients affected by acquired TTP for CD4+ T cells reactive against the previously identified FINVAPHAR and ASYLILIRD peptides derived from the CUB2 domain of ADAMTS13.
Study design
Peripheral blood samples
Peripheral blood samples from 2 patients with acquired TTP were included in this study. The study protocol was approved by the Medical Ethical Committee of the University Medical Center Utrecht in accordance with the Declaration of Helsinki. PBMCs of healthy volunteers were collected in accordance with Dutch regulations and after approval from the Sanquin Ethical Advisory Board in accordance with the Declaration of Helsinki. Blood was collected into heparinized tubes. PBMCs were isolated using Ficoll Plaque density centrifugation, frozen down in Dulbecco modified Eagle medium (Lonza, Breda, The Netherlands) supplemented with 50% fetal calf serum and 10% dimethylsulfoxide, and stored in liquid nitrogen until use.
Detection of antigen-specific CD4+ T cells
PBMCs from HLA-typed patients or healthy donors were thawed, washed, counted, and seeded in serum-free AIM-V medium (Life Technologies, Carlsbad, CA) in a 96-well plate at 2 × 106 cells per well. Cells were allowed to rest for 24 hours and were then stimulated with either 10 µg/mL FINVAPHAR peptide, CP_FINVAPHAR peptide, ASYLIRD peptide, CP_ASYILIRD peptide, full-length ADAMTS13 (FL-ADAMTS13), Staphylococcus aureus enterotoxin B (SEB) (1 µg/mL; Sigma-Aldrich, St Louis, MO), or AIM-V medium only; all conditions were supplemented with 1 µg/mL blocking antibody αCD40 pure (clone HB14; Miltenyi Biotec, Auburn, CA). Samples were stimulated for 24 hours and subsequently harvested and labeled with the Live/Dead Fixable Near-IR Dead Cell Stain kit (Life Technologies), anti-mouse CD3 Alexa Fluor 700 (clone 17A2; eBioscience, San Diego, CA), BUV395 mouse anti-human CD4 (BD Biosciences, San Jose, CA), CD14 QDot 800 (clone TüK4; Life Technologies), CD19 QDot 655 (clone SJ5-C1; Life Technologies), CD8 PerCp-Cy 5.5 (BD Biosciences), phycoerythrin mouse anti-human CD154 (BD Biosciences) and analyzed by flow cytometry (LSRFortessa; BD Biosciences). The gating strategy for these experiments is shown in supplemental Figure 1 (available on the Blood Web site).
The synthetic peptides FINVAPHAR (NAGGARLFINVAPHARIAIH), CP_FINVAPHAR (SPAISNAGGCRLKINTADHANAIHALATNM), ASYILIRD (EGANASYILIRDTHSLRTTA), and CP_ASYILIRD (EGANASYITIRPTFSLNTTA) used in this study were at least 90% pure as assessed by high-performance liquid chromatography and mass spectrometry; peptides were prepared and analyzed at the Peptide Core Facility at the Dutch Cancer Institute. The anchor residues involved in MHC-II binding were modified in the control peptides (underlined residues). Peptides were dissolved in dimethylsulfoxide and stored at −30°C. Recombinant human ADAMTS13 was obtained as described previously.17
Results and discussion
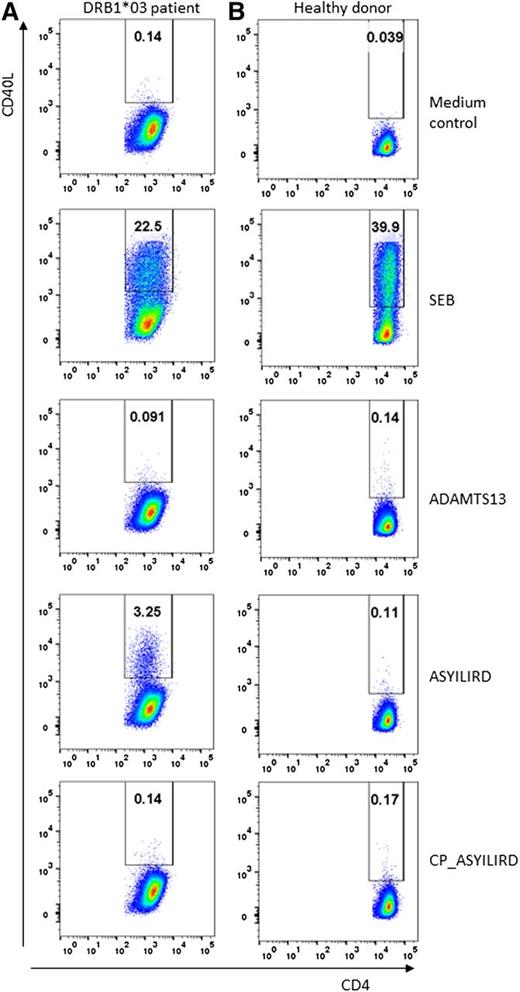
In vitro stimulation of PBMCs from either healthy donors or patients affected by TTP with FINVAPHAR- and ASYILIRD-containing peptides was analyzed by flow cytometry. PBMCs incubated with AIM-V medium only were used as a negative control. Stimulation with SEB was used as a positive control in our experiments.18 SEB is a superantigen crosslinking specific Vβ-chains (Vβ12, 14, 15, and 17) of the TCR with MHC-II.19 We first analyzed a sample collected during the acute phase of an HLA-DRB1*11–positive patient with acquired TTP. Stimulation with SEB resulted in an expression of CD40L on 25% to 40% of the CD4+ T cells from both this patient as well as a healthy control (Figure 1). Upon incubation with full-length ADAMTS13, a distinct population of CD40L-positive CD4+ T cells was observed (Figure 1). CD40L-positive CD4+ T cells were also observed upon incubation with the CUB2 domain peptide FINVAPHAR (Figure 1). The frequency of FINVAPHAR-positive cells was higher than observed for full length–ADAMTS13 (4.5% vs 2.6%). This may be due to the fact that ADAMTS13 needs to be processed by antigen-presenting cells in order to be presented on MHC-II. No CD4+ T cells recognizing a control FINVAPHAR-containing peptide (CP_FINVAPHAR) in which the anchor residues required for MHC-II binding were modified were identified in PBMCs from this patient (Figure 1). These results show that ADAMTS13 as well as FINVAPHAR-reactive CD4+ T cells are present in this patient during the acute phase of the disease. The patient analyzed suffered from relapsing TTP that was resolved following splenectomy. No ADAMTS13- or FINVAPHAR-reactive CD4+ T cells were observed in a sample obtained 2 years after splenectomy. We cannot exclude that tissue-resident CD4+ T cells recognizing the FINVAPHAR peptide are still present in this patient. No ADAMTS13- and FINVAPHAR-reactive CD4+ T cells were observed in PBMCs of HLA-DRB1*11–positive healthy individuals (Figure 1). We also addressed whether we could identify ADAMTS13-reactive CD4+ T cells in a peripheral blood sample obtained from an HLA-DRB1*03–positive patient during the acute phase of the disease. A distinct population of CD40L-positive CD4+ T cells was observed upon incubation with an ASYILIRD-containing peptide; 3.3% of the total number of CD4+ T cells responded to this peptide (Figure 2). We have previously shown that this peptide was presented on ADAMTS13-pulsed dendritic cells most likely in a HLA-DRB1*03–dependent manner.12 Stimulation with the corresponding control peptide (CP_ASYILIRD) in which the anchor residues were modified did not result in activation of CD4+ T cells (Figure 2). As expected, no ASYILIRD-reactive CD4+ T cells were observed in PBMCs derived of healthy individuals (Figure 2). In this patient, no activated CD40L+ T cells were observed upon incubation with ADAMTS13 (Figure 2). The absence of ADAMTS13-reactive CD4+ T cells may be due to insufficient processing of full length–ADAMTS13 under our experimental conditions. The CUB2 domain of ADAMTS13 contains an N-linked glycan at residue N135420 ; this glycan is closely located to the ASYILIRD peptide (residues A1355-D1362) and this might affect efficiency of presentation of the ASYILIRD peptide on MHC-II.
CD4+ T-cell response in an HLA-DRB1*11–positive patient with acquired TTP. (A) Analysis of PBMCs from an HLA-DRB1*11 patient in the acute phase of the disease. Incubations of PBMCs with AIM-V (medium control), SEB (positive control which activates a subset of CD4+ T cells by crosslinking MHC-II to TCRs with specific Vβ chains [Vβ12, 14, 15, and 17]),19 ADAMTS13 (10 µg/mL), FINVAPHAR-peptide (10 µg/mL), CP_FINVAPHAR peptide (10 µg/mL) with modified MHC-II anchor residues (negative control). CD40L is plotted on the y-axis whereas CD4 is plotted on the x-axis. The percentage of CD4+ T cells expressing CD40L is depicted in the upper right quadrant. (B) Analysis of PBMCs obtained from an HLA-DRB1*11 healthy volunteer. PBMCs were incubated as described for the sample depicted in the panel A column. PBMCs of this HLA-DRB1*11–positive healthy volunteer only respond to SEB stimulation and not to stimulation with ADAMTS13, FINVAPHAR, or CP_FINVAPHAR peptides. (C) Analysis of PBMCs derived of the same HLA-DRB1*11 TTP patient 2 years after splenectomy revealed no FINVAPHAR- or ADAMTS13-reactive CD4+ T cells. We acquired at least 0.5 × 106 events for each sample analyzed.
CD4+ T-cell response in an HLA-DRB1*11–positive patient with acquired TTP. (A) Analysis of PBMCs from an HLA-DRB1*11 patient in the acute phase of the disease. Incubations of PBMCs with AIM-V (medium control), SEB (positive control which activates a subset of CD4+ T cells by crosslinking MHC-II to TCRs with specific Vβ chains [Vβ12, 14, 15, and 17]),19 ADAMTS13 (10 µg/mL), FINVAPHAR-peptide (10 µg/mL), CP_FINVAPHAR peptide (10 µg/mL) with modified MHC-II anchor residues (negative control). CD40L is plotted on the y-axis whereas CD4 is plotted on the x-axis. The percentage of CD4+ T cells expressing CD40L is depicted in the upper right quadrant. (B) Analysis of PBMCs obtained from an HLA-DRB1*11 healthy volunteer. PBMCs were incubated as described for the sample depicted in the panel A column. PBMCs of this HLA-DRB1*11–positive healthy volunteer only respond to SEB stimulation and not to stimulation with ADAMTS13, FINVAPHAR, or CP_FINVAPHAR peptides. (C) Analysis of PBMCs derived of the same HLA-DRB1*11 TTP patient 2 years after splenectomy revealed no FINVAPHAR- or ADAMTS13-reactive CD4+ T cells. We acquired at least 0.5 × 106 events for each sample analyzed.
CD4+ T-cell response in an HLA-DRB1*03–positive patient with acquired TTP. (A) Analysis of PBMCs from an HLA-DRB1*03–positive patient in the acute phase of the disease. Incubations of PBMCs with AIM-V (medium control), SEB (positive control), ADAMTS13 (10 µg/mL), ASYILIRD peptide (10 µg/mL), CP_ASYILIRD peptide (10 µg/mL) with modified anchor residues (negative control). CD40L is plotted on the y-axis whereas CD4 is plotted on the x-axis. The percentage of CD4+ T cells expressing CD40L is depicted in the upper right quadrant. (B) Analysis of PBMCs obtained from an HLA-DRB1*03–positive healthy volunteer. PBMCs were incubated as described for the sample depicted in the panel A column. PBMCs of this HLA-DRB1*03–positive healthy volunteer only respond to SEB stimulation and not to stimulation with ADAMTS13, ASYILIRD, or CP_ASYILIRD peptides. We acquired at least 0.5 × 106 events for each sample analyzed.
CD4+ T-cell response in an HLA-DRB1*03–positive patient with acquired TTP. (A) Analysis of PBMCs from an HLA-DRB1*03–positive patient in the acute phase of the disease. Incubations of PBMCs with AIM-V (medium control), SEB (positive control), ADAMTS13 (10 µg/mL), ASYILIRD peptide (10 µg/mL), CP_ASYILIRD peptide (10 µg/mL) with modified anchor residues (negative control). CD40L is plotted on the y-axis whereas CD4 is plotted on the x-axis. The percentage of CD4+ T cells expressing CD40L is depicted in the upper right quadrant. (B) Analysis of PBMCs obtained from an HLA-DRB1*03–positive healthy volunteer. PBMCs were incubated as described for the sample depicted in the panel A column. PBMCs of this HLA-DRB1*03–positive healthy volunteer only respond to SEB stimulation and not to stimulation with ADAMTS13, ASYILIRD, or CP_ASYILIRD peptides. We acquired at least 0.5 × 106 events for each sample analyzed.
Altogether, we provide evidence for the presence of FINVAPHAR- and ASYILIRD-reactive CD4+ T cells in 2 patients who were analyzed in this study. We are currently exploring whether FINVAPHAR- and ASYILIRD-positive CD4+ T cells are also present in other patients with acquired TTP. Low levels of ADAMTS13 have previously been associated with an increased risk for relapse in patients with acquired TTP.21-23 In future studies, we will assess whether ADAMTS13-specific CD4+ T cells are still present in the periphery of patients with relapsing TTP.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Erik Mul, Simon Tol, and Mark Hoogenboezem for calibration and maintenance of the flow cytometers.
Authorship
Contribution: F.C.V. and F.d.H. performed the experiments; F.C.V. and J.V. wrote the manuscript; P.K. purified ADAMTS13; R.F. overlooked and arranged clinical aspects of this study and was responsible for sample collection; N.L. performed HLA typing; N.S. initiated the CD4+ T-cell experiments; A.W.T. setup the protocol for the assay used; and A.t.B. provided valuable input for this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for N.S. is Harvard Medical School Program in Cellular and Molecular Medicine, Boston Children’s Hospital, Boston, MA.
Correspondence: Jan Voorberg, Department of Plasma Proteins, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: j.voorberg@sanquin.nl.

![Figure 1. CD4+ T-cell response in an HLA-DRB1*11–positive patient with acquired TTP. (A) Analysis of PBMCs from an HLA-DRB1*11 patient in the acute phase of the disease. Incubations of PBMCs with AIM-V (medium control), SEB (positive control which activates a subset of CD4+ T cells by crosslinking MHC-II to TCRs with specific Vβ chains [Vβ12, 14, 15, and 17]),19 ADAMTS13 (10 µg/mL), FINVAPHAR-peptide (10 µg/mL), CP_FINVAPHAR peptide (10 µg/mL) with modified MHC-II anchor residues (negative control). CD40L is plotted on the y-axis whereas CD4 is plotted on the x-axis. The percentage of CD4+ T cells expressing CD40L is depicted in the upper right quadrant. (B) Analysis of PBMCs obtained from an HLA-DRB1*11 healthy volunteer. PBMCs were incubated as described for the sample depicted in the panel A column. PBMCs of this HLA-DRB1*11–positive healthy volunteer only respond to SEB stimulation and not to stimulation with ADAMTS13, FINVAPHAR, or CP_FINVAPHAR peptides. (C) Analysis of PBMCs derived of the same HLA-DRB1*11 TTP patient 2 years after splenectomy revealed no FINVAPHAR- or ADAMTS13-reactive CD4+ T cells. We acquired at least 0.5 × 106 events for each sample analyzed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/12/10.1182_blood-2015-10-668053/5/m_1606f1.jpeg?Expires=1769084739&Signature=2PnZi6dAPLlEndZqmI~COCKpxOJElURibpE-MmETvUhmSqfDRjwJKnAAv1Qg6SvGEnjCg0nI-h27gzz8agqqsdsOs56GtFkAYYaw5T7Ky~CgJ8PB9qlaNFJ9xxq-Qh6d~rd0PXSm8ckXkP-lo6Vu8tYr-8fN~BtQtFW~Is2MFpEJYW1ub0dvwpN3j1kz2QAisRhqSFJOtV~D2G58nooy1CTihpyRSgI8ZG48CTJpoGYNPmYFWYQuL03sbAlA0KmpO2NEuBNFruotjrhhOWVyRrhMM33zu7RK93RW1LcOIdacJCTWGdlj-StAaOf2vpdD7rWcIYbO~7Dv6gol4uJwTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)