Key Points
Fifty percent of TA-GVHD cases occur in patients who would not be predicted to be at risk for TA-GVHD by current guidelines for blood irradiation.
Donor lymphocytes whose HLA antigens are all shared by the recipient dominate in TA-GVHD cases, particularly in immune-competent recipients.
Abstract
Transfusion-associated graft-versus-host disease (TA-GVHD) is a rare complication of blood transfusion. The clinicolaboratory features of TA-GVHD and the relative contributions of recipient and component factors remain poorly understood. We conducted a systematic review of TA-GVHD reports. The HLA relationship between donor and recipient was classified as D = 0 when no donor antigens were foreign to the recipient vs D ≥ 1 when ≥1 donor antigen disparity occurred. We identified 348 unique cases. Criteria for component irradiation were met in 48.9% of cases (34.5% immune-compromised, 14.4% related-donor), although nonirradiated components were transfused in the vast majority of these (97.6%). Components were typically whole blood and red cells. When reported, component storage duration was ≤10 days in 94%, and 23 (6.6%) were leukoreduced (10 bedside, 2 prestorage, and 11 unknown). Among 84 cases with HLA data available, the category of D = 0 was present in 60 patients (71%) at either HLA class I or II loci and was more common among recipients without traditional indications for component irradiation. These data challenge the historic emphasis on host immune defects in the pathogenesis of TA-GVHD. The dominant mechanism of TA-GVHD in both immunocompetent and compromised hosts is exposure to viable donor lymphocytes not recognized as foreign by, but able to respond against, the recipient.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 426.
Disclosures
The authors, Associate Editor Mario Cazzola, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the features of components transfused in patients with transfusion-associated graft-versus-host disease (TA-GVHD), based on a systematic review of TA-GVHD reports.
Distinguish the human leukocyte antigen features of donors implicated in TA-GVHD and their respective recipients.
Discuss the relative contributions of recipient and component factors to TA-GVHD.
Release date: July 16, 2015; Expiration date: July 16, 2016
Introduction
Transfusion-associated graft-versus-host disease (TA-GVHD) is a rare, usually fatal, complication of blood transfusion wherein donor lymphocytes in a transfused blood component mount an immunodestructive response against recipient tissues.1 Patients typically present with a constellation of fever, rash, gastrointestinal symptoms, liver injury, and hypoproliferative pancytopenia. Mortality in TA-GVHD has been estimated to be between 90% and 100%.2-5 According to criteria devised by the National Health and Safety Network (NHSN), the diagnosis of TA-GVHD is based on a combination of characteristic clinical findings and a tissue biopsy consistent with GVHD, with imputability established via the demonstration of leukocyte chimerism, specifically donor lymphocytes in recipient tissue (Table 1).6
The predominant factors leading to TA-GVHD have yet to be fully elucidated. Donor lymphocytes were first implicated in GVHD in the 1950s.7 An inability of the host’s immune system to recognize and/or eliminate foreign donor cells in allogeneic blood components was subsequently identified as a key mechanism.8 Some of the first reported cases of GVHD attributed to transfusion occurred in immunocompromised infants and fetuses,9,10 and are likely to have inspired the historical attribution of TA-GVHD to immune defects of the transfusion recipient11 ; however, cases of a frequently fatal “postoperative erythroderma” syndrome concentrated in Japan were reported earlier, without initially identifying the significance of transfusions received by the subjects.12
Early initiatives aimed at preventing TA-GVHD involved blood component irradiation for those recipients perceived to have impaired immunity4,13 ; this population is still emphasized in recommendations regarding blood irradiation, although precisely which diagnoses warrant irradiation remains unresolved between experts and institutions.14,15
It was later realized that HLA similarity between donor and recipient, and specifically, donor homozygosity for an HLA haplotype for which the recipient was heterozygous, was a likely explanation for the cases occurring within and beyond Japan in transfusions from related donors16-18 ; recommendations to irradiate related-donor or HLA-matched blood components followed.18-20 Such HLA relationships lead to an ability of donor lymphocytes to evade HLA-mediated immune detection by the host, but a preserved ability of donor lymphocytes to recognize and react to host tissues. Host immune deficits, component characteristics, and the donor-recipient HLA relationship are all thought to play a role in the development of TA-GHVD, although their relative contributions remain unclear.
Irradiation of cellular blood components, the best-established intervention to prevent TA-GVHD, may generate operational costs, time delays (in particular if irradiation is not done in-house), and in the case of red cells, damages transfused cells.21-23 Therefore, the identification of cases warranting irradiation remains important. Current guidelines for irradiation of cellular blood components are largely driven by the immune status of the recipient, with the common exception of the irradiation of HLA-matched platelet transfusions.24 The list of relevant diagnoses has been assembled from case reports and small case series, many of which did not investigate or report the HLA relationship between donor and recipient.
To better understand the relative importance of patient (underlying immunodeficiency) or component (donor HLA similarities, component storage time, leukoreduction) factors for the prevention of TA-GVHD and to evaluate the NHSN criteria, we undertook a systematic review of all reported cases of TA-GVHD in the medical literature. This study is intended to inform evidence-based guidelines for prevention of TA-GVHD.
Methods
This review was registered with the University of York PROSPERO registry of systematic reviews; a protocol is available online at http://www.crd.york.ac.uk/prospero/search.asp (registration #CRD42013006125).
Any published case that was labeled as TA-GVHD on plausible grounds was included for systematic review; cases in which the diagnosis was ultimately disproven were excluded. Cases of GVHD following stem cell transplant were excluded, unless they were demonstrated to be caused by transfused blood components rather than the stem cell donor graft. Cases of hemolytic passenger lymphocyte syndromes and cases attributed to granulocyte transfusions were excluded.
Literature search
A comprehensive search was undertaken of MEDLINE (Pubmed/OVID), EMBASE (OVID), the Cochrane library, Web of Science, British Medical Journal case reports, and the International Society of Blood Transfusion proceedings (supplemental Appendix available on the Blood Web site). No restrictions were placed on language. Databases were searched from their inception until September 16, 2013.
Titles and abstracts of all items identified by the search were reviewed by 2 independent reviewers and designated as relevant (or not) to the study. Items deemed irrelevant by both reviewers were excluded; all other items (deemed potentially relevant by one or both reviewers) were included for detailed review. Review articles, book chapters, or other general resources identified in the literature search whose content was thought to be potentially relevant were included; these were then screened by a reviewer for additional cases, which included a search of reference lists or bibliographies.
References for which the complete article could not be accessed or purchased through our institutional libraries were excluded. Efforts were made to translate non-English articles; we obtained translator services for French, German, Italian, Spanish, and Japanese.
Data abstraction and missing data
Data collection was undertaken by the authors using a standardized data collection form. Whenever possible, data entries represented analyzable input items from a dropdown list. All reviewers were trained in the use of the data collection form. Where data were missing or not reported, no data were substituted unless they could be legitimately inferred from available details (eg, subjects’ whose spleens were described in an autopsy report were concluded not to have undergone prior splenectomy). When critical information was not reported, authors of original publications were contacted. Data collected included patient demographics and diagnoses, details regarding the transfusion event(s) and blood component characteristics, clinicolaboratory features of the TA-GVHD presentation and outcome, and results of HLA and chimerism studies.
Definitions
The Centers for Disease Control and Prevention NHSN hemovigilance case definitions and criteria for imputability were applied (Table 1); for the case definitions, we deemed the clinical criteria met if any of the listed clinical features were present. Study definitions were as follows. Rash was any acute dermatologic lesion. Diarrhea was deemed present if stated as such by the authors or if an increased frequency or volume or watery consistency of stools was described. Elevated liver enzymes was any increase (above the upper limit of normal as specified in the publication) in aminotransferases, alkaline phosphatase, and/or bilirubin. Pancytopenia was deemed present if explicitly stated by authors or if a decline in 3 cell lines (leukocytes, red blood cells [RBCs], and platelets) to below the lower limit of normal was reported. Chimerism was defined as the demonstrable presence of ≥2 cell lines with unique genetic material, documented by analysis of polymorphic DNA sequences (ie, restriction fragment length polymorphisms, tandem repeat loci), karyotyping, or other means.
We applied current guidelines for irradiation of blood components (Massachusetts General Hospital, Boston, MA; Sunnybrook Health Sciences Centre, University Health Network, Hospital for Sick Children, Toronto, ON, Canada) to classify recipients based on the presence or absence of a recommended indication for irradiation (Table 2).
HLA typing
We collected all available HLA data where typing was done for ≥1 HLA antigen, by serological or molecular methods, for both the recipient and the donor (or component). Where both molecular and serologic typing was performed, the molecular type was recorded. Where typing for a particular HLA antigen was done only in donor or recipient but not both, this typing was excluded. HLA donor-recipient relationships were defined for HLA class I and class II antigens separately, as D = 0, where the donor profile lacked any identifiably foreign antigen types with respect to the recipient, or as D ≥ 1, where donor cells expressed ≥1 HLA antigen foreign to the recipient. We included in the D = 0 category all cases where no foreign donor antigen was identified, irrespective of the presence or absence of recipient antigens disparate from the donor. Examples of the application of this classification scheme are shown in Table 3.
Data analysis
Following data extraction, the compiled database was screened for duplicate cases. This was done by identifying case reports on subjects with the same age, sex, country of origin, or author(s).
Categorical variables are described as percentages, normally distributed variables as means with standard deviations, and nonnormally distributed variables as medians with interquartile ranges (IQRs). All comparisons were 2-sided with α = 0.05.
Results
Search results
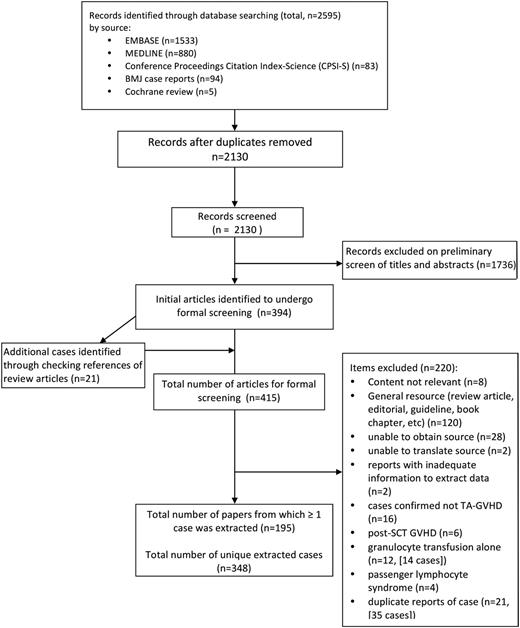
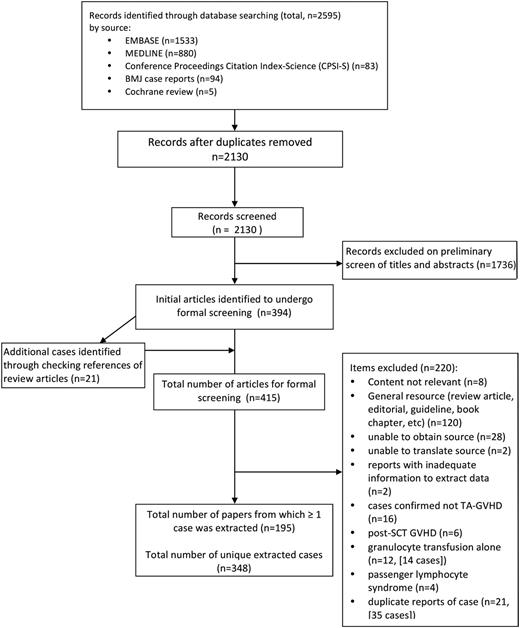
After removing duplicates, 2130 citations discovered by the search were examined by 2 independent reviewers, with 394 identified as publications of interest for complete review. Through checking all references from review articles found in our initial search, an additional 21 publications were identified for a total of 415 publications. Of these, 220 did not report ≥1 extractable cases. The remaining 195 publications, describing 348 unique cases, form the basis of our analysis (Figure 1; full list of publications available in supplemental Appendix).
The most common countries of origin were Japan (n = 146), the United States (n = 50), the United Kingdom (n = 36), Turkey (n = 18), Lebanon (n = 15), Germany (n = 15), and India (n = 12). Six or fewer cases were reported from Argentina, Australia, Austria, Canada, China, Denmark, Finland, France, Iran, Israel, Italy, Netherlands, Pakistan, Poland, Portugal, Scotland, Spain, Switzerland, and Tasmania.
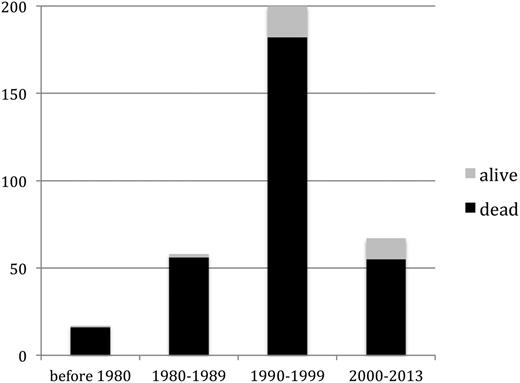
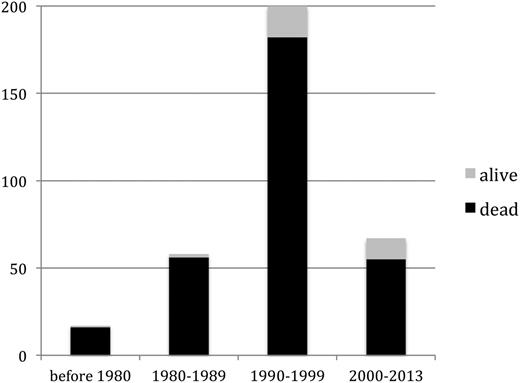
Seventeen cases of TA-GVHD occurred (or were reported) between 1966 and 1979, 68 cases between 1980 and 1989, 197 cases between 1990 and 1999, and 66 cases between 2000 and 2013.
Patients
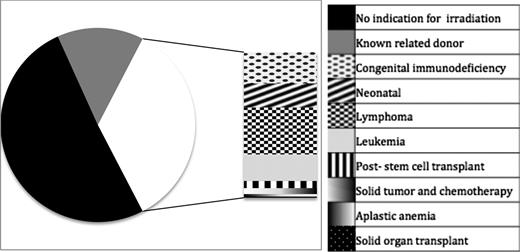
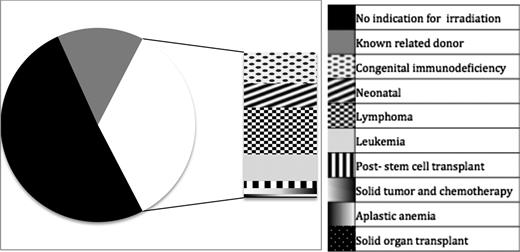
Overall, 61.6% of those diagnosed with TA-GVHD were men. Median age at diagnosis was 58 years (IQR, 18-68 years; range, intrauterine to age 97. The most frequent underlying diagnoses or indications for transfusion were noncardiac surgery (n = 81, 23.3%), cardiac surgery (n = 71, 20.4%), hematologic malignancy (n = 67, 19.3%), and congenital immunodeficiency (n = 25, 7.2%; Figure 2).
Diagnoses in patients with TA-GVHD. Two hundred twenty-seven (65.2%) patients had no diagnosis conferring an immunocompromised state, whereas the remaining 121 (34.8%) had either a congenital or acquired immune deficiency. The stacked bar illustrates the various immune defects reported in cases of TA-GVHD. Nine patients had received purine analogs (8 fludarabine and 1 cladrabine) and 2 had received anti-thymocyte globulin; all of these had alternate diagnoses (eg, lymphoma or prior stem cell transplant).
Diagnoses in patients with TA-GVHD. Two hundred twenty-seven (65.2%) patients had no diagnosis conferring an immunocompromised state, whereas the remaining 121 (34.8%) had either a congenital or acquired immune deficiency. The stacked bar illustrates the various immune defects reported in cases of TA-GVHD. Nine patients had received purine analogs (8 fludarabine and 1 cladrabine) and 2 had received anti-thymocyte globulin; all of these had alternate diagnoses (eg, lymphoma or prior stem cell transplant).
According to current guidelines for irradiation of blood components, of 348 cases, 121 (34.8%) occurred in recipients who would warrant blood component irradiation based on underlying diagnosis. Fifty additional patients (14.4%) received blood components from a known related donor. The remaining 177 cases (50.9%) lacked all recipient and donor factors addressed in current guidelines for component irradiation.
Presentation and TA-GVHD diagnosis
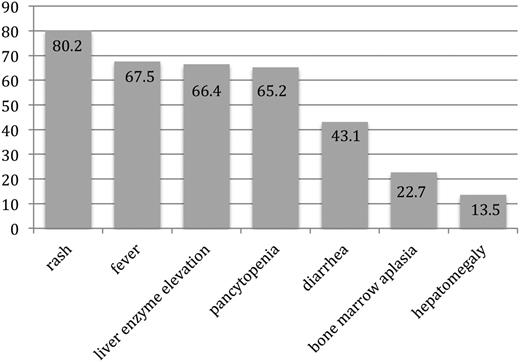
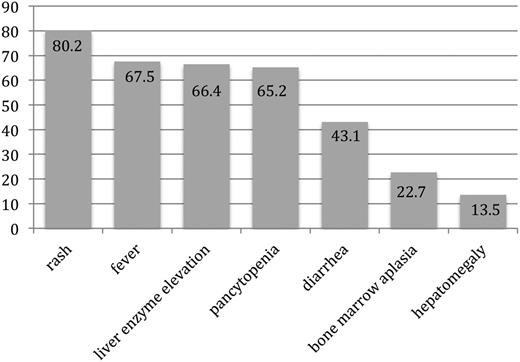
Among the 245 patients in whom the specific time of symptom onset relative to transfusion was reported, the first symptom of TA-GVHD occurred a median of 11 days (IQR, 8-14 days; range, 1-198 days) from the implicated transfusion. In 9 cases, symptom onset occurred at >6 weeks from the implicated transfusion. When considering the clinical signs and symptoms according to the NHSN definition, rash was reported in 279 (80.2%), fever in 235 (67.5%), liver enzyme elevation in 231 (66.4%), pancytopenia in 227 (65.2%), diarrhea in 150 (43.1%), bone marrow aplasia in 79 (22.7%) or hypocellularity in 60 (17.2%), and hepatomegaly in 47 (13.5%) (Figure 3). An average of 4 (standard deviation, 1) of the 7 findings were present. All 7 manifestations were reportedly present in only 1 patient.
Proportion of patients with reported symptoms and signs of TA-GVHD included in National Health and Safety Network case definition. Patients with TA-GVHD presented with an average of 4 of the 7 clinical findings included in the NHSN case definition.
Proportion of patients with reported symptoms and signs of TA-GVHD included in National Health and Safety Network case definition. Patients with TA-GVHD presented with an average of 4 of the 7 clinical findings included in the NHSN case definition.
The NHSN case definition criteria for a definitive diagnosis include both clinical and histologic findings consistent with TA-GVHD, occurring between 2 days and 6 weeks from transfusion of the implicated component. In the setting of a clinical syndrome consistent with the diagnosis but without a biopsy to support the diagnosis, the case is deemed probable TA-GVHD (Table 1). Because precise timing of symptom onset was often not documented, we elected to disregard this aspect of the case definitions for the classification of cases. Using this modified classification system, 236 (67.8%) patients met criteria for definitive diagnosis and 49 (14.1%) had a probable diagnosis of TA-GVHD.
Biopsies were acquired in 240 (69.0%) of cases overall. Among these, skin was biopsied in the majority (n = 230, 95.0%), either alone (n = 198) or along with other tissues (n = 32). Other tissues biopsied included gastrointestinal tract, liver, and lymph nodes. Leukocyte chimerism was identified in 102 (29.3%) cases.
Implicated components
The component implicated in TA-GVHD was identified in 248 cases (71.3%): RBCs in 133 cases (38.2%); whole blood (WB) in 92 cases (26.4%); platelets in 20 cases (5.7%); buffy-coat product in 2 cases (0.6%); and fresh plasma in 1 case. In the remaining 100 (28.7%) cases, the causal component was either not specified or not identified among several potentially responsible components. Component storage time was reported in 158 cases (45.4%). Of these, the implicated component was either described as fresh or ≤10 days old in 148 cases (93.7%). Ten cases (6.3%) reported a storage time of 11 to 14 days, with no cases implicating components stored for >2 weeks.
Leukoreduction status was reported in 135 cases (38.8%). Of these, the implicated component was leukoreduced in 23 cases (17.0%; 6.6% of all cases; 10 bedside, 2 prestorage, and 11 not specified). The component was irradiated in 5 cases (1.4%): in 2 cases, 25 cGy was reportedly delivered; in 1 case, 15 cGy was used; in 1 case, an in-house X-ray machine was used for irradiation; and in another case, the irradiation procedure was not detailed.
In many reports, the number of transfused units was not specified; therefore, average exposure volumes and whether these differed between patient groups (eg, surgical vs nonsurgical patients) could not be evaluated accurately.
HLA
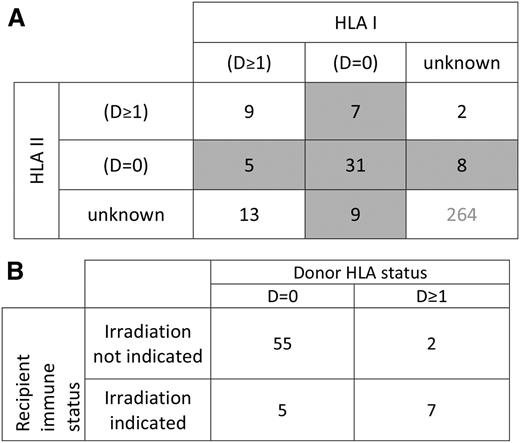
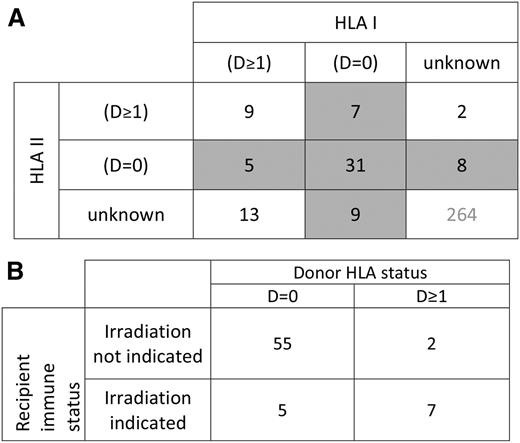
HLA typing of recipient and donor at ≥1 HLA loci, by serological or molecular techniques, was available for 84 cases (24.1%, 52 both HLA class I and I information, 22 class I only, and 10 HLA class II only). Among patients with HLA data available, 20 (24%) had an underlying diagnosis warranting irradiation by current standards, whereas 64 (76%) did not. Those with an accepted indication for irradiation included 11 patients with acquired immunodeficiency states (7 lymphoma, 3 acute myeloid leukemia, and 1 acute lymphoblastic leukemia), 7 with a congenital immunodeficiency syndrome, 1 premature infant, and 1 case involving intrauterine transfusion. The category of D = 0 was found in 47 of 74 (64%) cases with reported class I typing and 44 of 62 (71%) cases with reported class II typing. Overall, 60 of 84 (71%) cases with HLA typing were D = 0 at either HLA I, HLA II, or both (Figure 4A). There were 9 cases in which the category of D = 0 could be ruled out for both HLA I and II. In the remaining 15 cases, the category of D = 0 at either HLA I or II could not be definitively ruled in or out based on reported data. When considering those in whom the presence or absence of D = 0 could be definitely assigned, D = 0 at either HLA class I or class II was present in 55 of 57 (96%) of recipients without a diagnosis representing an indication for blood component irradiation, and 5 of 12 (42%) of those with an indication for irradiation (risk ratio, 2.3; P < .0001; Figure 4B).
Reported HLA relationships in cases of TA-GVHD. (A) Donor-recipient relationships for HLA class I and class II in cases of TA-GVHD. D = 0 indicates an absence of donor antigens foreign to the recipient. D ≥ 1 indicates the presence of ≥1 foreign donor antigens. Shaded areas indicate cases in which an absence of foreign donor antigens was reported at either HLA class I or class II loci. (B) Among those in whom the presence or absence of D = 0 could be definitively determined, donor stealth (D = 0) was present in 55 of 57 (96%) of those without an indication for irradiation. In contrast, donor disparity (D ≥ 1) was present in 58% (7 of 12) of patients with an indication for blood irradiation. (P < .0001).
Reported HLA relationships in cases of TA-GVHD. (A) Donor-recipient relationships for HLA class I and class II in cases of TA-GVHD. D = 0 indicates an absence of donor antigens foreign to the recipient. D ≥ 1 indicates the presence of ≥1 foreign donor antigens. Shaded areas indicate cases in which an absence of foreign donor antigens was reported at either HLA class I or class II loci. (B) Among those in whom the presence or absence of D = 0 could be definitively determined, donor stealth (D = 0) was present in 55 of 57 (96%) of those without an indication for irradiation. In contrast, donor disparity (D ≥ 1) was present in 58% (7 of 12) of patients with an indication for blood irradiation. (P < .0001).
TA-GVHD treatment and outcomes
The most common treatment approach was administration of immunosuppressive or immunomodulatory medications, which was reported in 175 cases (50.3%). Eight patients (2.3%) underwent stem cell transplant (SCT).
With 312 fatalities, 89.7% of patients died, at a median of 24 days (IQR, 19-32 days) after the implicated transfusion, whereas 29 patients (8.3%) recovered (Figure 5). Among those not undergoing SCT, 7.6% survived. The outcome was not reported in the remaining 7 (2.0%). Death was associated with older age (median, 59 years; IQR, 20-69 in those who died vs 35 years, IQR 5-50, in survivors; P < .01); WB (28.5% vs 6.9%; P = .012); nonleukoreduced implicated product (94.9% vs 79.3%; P = .013); and short storage durations classified as fresh or ≤48 hours (26.1% vs 6.9%; P = .03). Survivors disproportionately had indications for irradiation (58.6% vs 32.7%, P = .011) and were rescued by SCT (10.3% vs 1.6%; P = .046; Table 4).
Survivors of TA-GVHD. A higher proportion of patients with TA-GVHD survived in more recent time periods (P = .04), although this subset remained a small minority of cases in all eras.
Survivors of TA-GVHD. A higher proportion of patients with TA-GVHD survived in more recent time periods (P = .04), although this subset remained a small minority of cases in all eras.
Impact of era
Patient and component characteristics largely did not differ in cases occurring before 2000 compared with those occurring in or after 2000 (data not shown). More patients in the post-2000 era underwent biopsy (92.4% vs 63.5%; P < .001), and a larger proportion survived (19.7% vs 8.2%; P = .02).
Discussion
We report the first systematic review of all cases of TA-GVHD in the medical literature. We found that most patients with TA-GVHD did not have an underlying diagnosis conferring immune compromise, with approximately half not having qualified for irradiated blood components according to current guidelines, based predominantly on patient diagnosis. The majority of cases in our review were attributed to cellular, nonleukoreduced, nonirradiated components that were stored for <10 days. Rare cases that occurred despite irradiation were, in several cases, because of technical deviations from modern standards. In the analysis of donor-recipient HLA relationships, the absence of foreign antigen expression with respect to the recipient (at either HLA class I or class II loci) was identified in 71% of cases.
This is the largest compilation of TA-GVHD cases to date, including nearly all published cases spanning 5 decades and 26 countries.
Our findings support the notion that TA-GVHD is principally attributable to viable donor lymphocytes present in the transfused component. Nearly all cases were attributed to cellular components stored for ≤10 days, with only 10 cases from components stored for 11 to 14 days. The United Kingdom’s hemovigilance reporting system (Serious Hazards of Transfusion [SHOT]) has supported the hypothesis that the number of lymphocytes is also relevant in TA-GVHD, by demonstrating a significant reduction in the risk of TA-GVHD after the implementation of leukoreduction.25 Although this association may be attributed to the concurrent improvement in adherence to irradiation policies, there have been >1000 lapses in the latter practice recorded in the SHOT database, and no cases of have been TA-GVHD reported (Paula Bolton-Maggs, verbal communication, May 2014). However, with 23 cases in our series having occurred after transfusion of leukoreduced components, our data suggest that leukoreduction alone is inadequate to prevent TA-GVHD in all instances; of note, leukoreduction techniques fell short of modern standards in ≥10 of these cases (bedside leuko-filtration), and prestorage technique could only be ascertained in 2 cases.26 Thus, a majority of these breakthrough cases, and the apparent inadequacy of leukoreduction to prevent TA-GVHD, may reflect process limitations of historic filtration techniques and may not apply to modern prestorage leukoreduction.27,28
We found a single case in which plasma was implicated, and this was fresh (never-frozen) plasma,29 which contains viable leukocytes and is not used in modern practice. Our data thus support that plasma and its fractionated products need not be irradiated.
Interestingly, our cohort included more men than women. One possible explanation for this is protection conferred through the immune response to prior pregnancies in parous women. Indeed, this sex imbalance was greater in adults, with males comprising 53% of those <12 years of age compared with 63% of those >12 years of age. Parity was rarely reported and should be included in future case documentation (Table 5).
That the vast majority of cases in our series were attributed to blood components of short (≤10 days) storage duration and that whole blood represented a substantial proportion of cases raise the question of whether these component features warrant routine irradiation. Whole blood is still regularly transfused in military settings, and several ongoing trials30-33 are exploring whether the use of fresher blood components or whole blood result in more favorable outcomes; if this research ultimately drives changes in practice and preferential use of such products, it will be critical to define and quantify the risk of TA-GVHD posed by fresh components and determine whether and how it should influence irradiation practices.
Our findings may also be taken to challenge the prevalent practice-guiding notion that immune status of the recipient is the main determinant of susceptibility to TA-GVHD. The majority of cases in our series involved transfusions in immunocompetent hosts. Although immunocompromised recipients comprised a considerable proportion in our cohort, the latter appeared no more enriched with such patients than other populations requiring transfusions,34 (Table 6), and the immune-compromised proportion did not change in successive eras, with presumed more prevalent application of irradiation policies and universal leukoreduction. Our findings question whether, or to what degree, underlying immune deficiencies contribute to susceptibility to TA-GVHD and to what degree these are incidental surrogates of exposure opportunities to other drivers of risk.
In addition to recipient immune compromise, an ability of donor lymphocytes to evade host immune detection, on the basis of shared HLA antigens has been proposed as a pathogenic mechanism in TA-GVHD.1,16-18,35 This circumstance was captured in our D = 0 category, which was present in 71% of those with HLA data available and in nearly all TA-GVHD cases in immunocompetent recipients. The proportion of D = 0 among immune-compromised hosts (42%) was also considerably higher than what was expected by chance alone in an unselected population of donor-recipient pairs. Thus, covert HLA types appear to play an important role in the development of TA-GVHD in recipients with all levels of immune function, as proposed by others,36 albeit possibly to a greater degree in those without impaired immunity. Despite 1000 cases of inadvertent transfusion of nonirradiated components to immunocompromised recipients warranting this measure, SHOT investigators found no cases of TA-GVHD, providing indirect evidence that immunodeficiency alone may not be sufficient grounds for TA-GVHD. The relatively high incidence of TA-GVHD in genetically homogeneous populations such as Japan, compared with countries with greater degrees of HLA heterogeneity, further supports the dominance of this mechanism.35,37 However, without systematic HLA typing in all cases of TA-GVHD, the precise contributions of permissive immune systems and HLA relationships remain unclear, and we acknowledge potential biases introduced by the selective undertaking of HLA evaluations. Our findings highlight the importance of complete investigation of HLA in every case of TA-GVHD.
A further consideration is whether HLA relationships are best considered as a dichotomy (a complete absence of foreign donor HLA types vs the presence of any foreign donor HLA types) or whether there exists a spectrum of HLA disparity and stealth, which predisposes to TA-GVHD to varying degrees. Interestingly, in several cases designated as D ≥ 1 in our series, the donor nevertheless harbored only few and subtle differences in HLA antigens compared with those present in the recipient, therein underestimating the true extent of potential host tolerance.3,38-44 Although D = 0 represents the ultimate surveillance escape, it is possible that nonrecognition is achievable by other variations.
This work was subject to several limitations. Not all cases of TA-GVHD have been clinically recognized and reported, and such cases likely reflect a publication bias. As such, this comprehensive assembly is but a subset of all cases, whose demographic, clinical, and laboratory features may thus be skewed and not be entirely representative. Cases of lesser severity, with atypical or limited features, and occurrences in diagnostically underresourced areas, may never be reported. Even among peer-reviewed cases, a great proportion lack diagnostic biopsies, and HLA typing and chimerism studies are evidently pursued in only a minority of cases. Numerous reports lacked pertinent information on the patient, component, or clinical circumstance. The inclusion of reports from as early as 1967 implies that diagnostic testing, terminology, and scientific paradigms contained in many reports are now considered outdated. To ensure that future reporting is able to inform the scientific and medical communities, we propose a set of data that should be collected and reported where possible in all cases of suspected or confirmed TA-GVHD (Table 5). Finally, in several instances, publications were irretrievable or beyond the available language translation resources.
Deriving an understanding TA-GVHD has proved challenging since its original description. Further systematic tracking of large numbers of patients with immune compromise inadvertently receiving nonirradiated components might continue to lend weight to the notion that immune status plays a secondary role in the development of TA-GVHD and possibly that immunodeficiency alone is insufficient for the development of TA-GVHD. We propose that any patient with features suggestive of TA-GVHD should undergo thorough and systematic evaluation. A rigorous and consistent standard of evaluation is needed to confirm our findings, grant objective insights into responsible mechanisms and their relative contribution to the development of TA-GVHD, and inform scientifically sound policies regarding blood component irradiation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Connie Colavecchia, Ana Lima, Nellie Castilla, and Magda Wojda for the kind offer to translate the Italian, French, Spanish, and Polish articles and Dawn Dawkins and Regina Mejia for obtaining publications from the literature search.
Authorship
Contribution: J.C., W.S.D., C.M.C.-G., Y.L., A.K.K., I.K., and J.O. contributed to the original concept; all authors contributed to study design, data collection, and analysis; I.K. and J.C. drafted the manuscript; and all authors provided major intellectual contributions to the manuscript, reviewed and revised its content, and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.T. is Department of Cardiovascular Surgery, Kyoto University, Kyoto, Japan.
Correspondence: Jeannie Callum, Sunnybrook Health Sciences Centre, B204, 2075 Bayview Ave, Toronto, ON, Canada M4N 3M5; e-mail: jeannie.callum@sunnybrook.ca.
References
Author notes
I.K. and J.O. contributed equally to this work.