Abstract
Introduction: There are few effective treatment options available for patients (pts) with myelofibrosis (MF). Pts with thrombocytopenia, a risk factor for shorter overall survival, have poorer prognosis (Gangat, J Clin Oncol 2010). Pacritinib is a kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CFSR1 and has demonstrated minimal myelosuppression in clinical trials (Hart, Leukemia 2011; Komrokji, Blood 2015; Mesa, ASCO 2015). In the phase III open-label PERSIST-1 trial, a significantly greater proportion of pts treated with pacritinib achieved spleen volume reductions (SVR) ≥35% vs BAT (ITT: 19.1% vs 4.7%, p=0.0003; pts evaluable at baseline and Week 24: 25.0% vs 5.9%, p=0.0001; Mesa, ASCO 2015). This analysis examines pt responses across subgroups.
Methods: Pts naive to treatment with JAK inhibitors were randomized 2:1 to receive oral pacritinib 400 mg once daily or BAT. Stratification factors included DIPSS risk status and baseline platelet count. Pts were eligible who had DIPSS Intermediate (Int)-1, Int-2, or High risk disease; absolute neutrophil count >500/µL; palpable splenomegaly ≥5 cm; and baseline Total Symptom Score (TSS) ≥13 using the Modified MPN Symptom Assessment Form Total Symptom Score (MPN-SAF TSS and TSS 2.0). There was no restriction on baseline platelets or hemoglobin (Hgb) levels. The primary endpoint was the proportion of pts achieving SVR ≥35% at Week 24 by centrally-reviewed MRI or CT and the secondary endpoint was the proportion of pts achieving ≥50% reduction in TSS from baseline at Week 24 using 6 symptoms from the MPN-SAF TSS. Pt responses were analyzed by baseline risk factors for MF including platelet counts (<50,000/µL vs ≥ 50,000/µL and <100,000/µL vs ≥100,000/µL), sex, age (≥65 y vs <65 y), JAK2V617F mutation status (positive vs negative), baseline MF diagnosis (primary MF [PMF] vs secondary MF), reticulin and collagen fibrosis staging (> 1 vs ≤1), TSS (≥20 vs <20), white blood cell count (>25×109/L vs ≤25×109/L), peripheral blasts (≥5% vs <5%), Hgb (<10 g/dL vs ≥10 g/dL), transfusion dependency by Gale criteria (Y vs N), time from diagnosis (<12 mos vs ≥12 mos), ECOG PS (2-3 vs 0-1), and bone pain (>3 vs ≤3). In multivariate logistic regressions, the odds of SVR ≥35% and TSS reduction ≥50% at Week 24 were modeled as a function of prognostic factors for MF and adjusted for treatment (pacritinib vs BAT). Results for the 6 symptoms common to both TSS versions are reported.
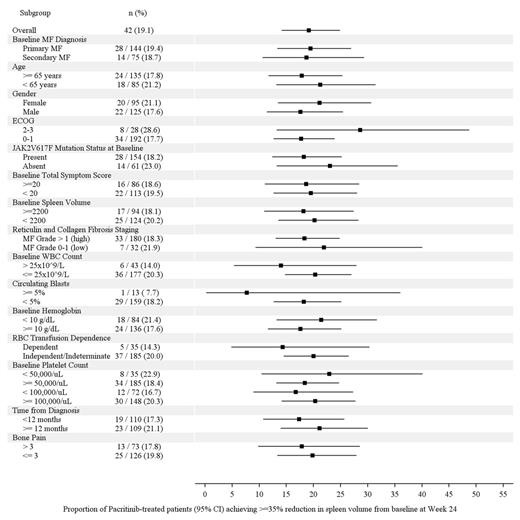
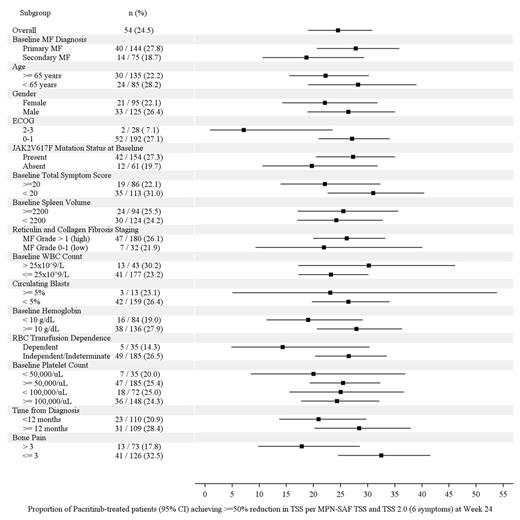
Results: 327 pts were enrolled and randomized (pacritinib: 220, BAT: 107). Overall, 62% of pts had PMF; 32% had baseline platelets <100,000/µL and 16% had <50,000/µL; 75% were positive for JAK2V617F. After a median follow-up of 8.4 months, treatment with pacritinib resulted in consistent rates of SVR across subgroups (Figure 1). When comparing vs BAT, the greatest differences in SVR ≥35% rates between treatment arms were observed in pts with baseline platelets <50,000/μL (+22.9% vs BAT), JAK2V617F-negative pts (+23.0% vs BAT) and those aged <65 y (+21.2% vs BAT). Improvements in TSS (TSS and TSS 2.0 reduction ≥50%) were also consistent for pts receiving pacritinib (Figure 2). By multivariate analysis, SVR ≥35% was significantly correlated only with ECOG PS ≥2 (odds ratio [OR]=2.97, p=0.030) and TSS reduction ≥50% was significantly correlated only with bone pain >3 (OR=0.35, p=0.004).
Conclusions: Treatment with pacritinib resulted in consistent rates of SVR ≥35% and TSS reduction ≥50% irrespective of baseline characteristics. Comparisons vs BAT were favorable for all patient subpopulations examined for both endpoints. These results support the use of pacritinib across all intermediate- or high risk MF pt subgroups analyzed.
Proportion of Patients Receiving PAC who Achieved ≥35% SVR from baseline to Week 24 (95% CI)
Proportion of Patients Receiving PAC who Achieved ≥35% SVR from baseline to Week 24 (95% CI)
Proportion of Patients Receiving PAC who Achieved ≥50% TSS reduction (6 common symptoms in MPN-SAF TSS and MPN-SAF TSS 2.0) from baseline to Week 24 (95% CI)
Proportion of Patients Receiving PAC who Achieved ≥50% TSS reduction (6 common symptoms in MPN-SAF TSS and MPN-SAF TSS 2.0) from baseline to Week 24 (95% CI)
Vannucchi:Shire: Speakers Bureau; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxalta: Membership on an entity's Board of Directors or advisory committees. Off Label Use: This abstract discusses off-label use of pacritinib. Mesa:Gilead: Research Funding; NS Pharma: Research Funding; Incyte Corporation: Research Funding; Genentech: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Promedior: Research Funding; Pfizer: Research Funding; CTI Biopharma: Research Funding. Cervantes:Novartis: Consultancy, Speakers Bureau; Sanofi-Aventis: Consultancy; CTI-Baxter: Consultancy, Speakers Bureau. Prasad:BIOGEN IDEC: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Speakers Bureau. Elinder:Celgene: Consultancy. Recher:Sunesis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Chugai: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. te Boekhorst:CTI Biopharma: Consultancy; Novartis: Consultancy. Somervaille:Novartis Pharmaceuticals Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees. Dean:CTI Biopharma: Employment, Equity Ownership. Harrison:Shire: Speakers Bureau; Gilead: Honoraria; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.