Abstract
Introduction
High dose chemotherapy followed by autologous peripheral blood stem cell transplant (APBSCT) is the current standard of care for myeloma patients younger than 70 years of age with good performance status. Busy haematology centres have severe pressure on bed availability which can limit their ability to deliver multiple cycles of intensive chemotherapy on time. G-CSF use in APBSCT has been shown to hasten neutrophil engraftment and to shorten the number of days of febrile neutropenia. In our centre we have had experience of using pegfilgastrim (day +1 post high dose therapy), lenograstim(day+7 post high dose therapy) or no G-CSF. We reviewed these cohorts and compared engraftment kinetics, inpatient stay, progression free survival(PFS) and overall survival(OS).
Patients and methods
This retrospective study included 142 (M: 94; F: 48) patients who had APBSCT for myeloma between January 2006 and March 2014. Patients had induction treatment followed by autologous peripheral blood stem cell (PBSC) mobilisation with G-CSF alone or by cyclophosphamide (3g/m2) + G-CSF schedule. One hundred thirteen patients received first APBSCT and twenty nine received second APBSCT with high dose melphalan (200 mg/ m2) conditioning and the day of stem cell re-infusion was termed day 0. Statistical analysis was carried out using SPSS 23 for Windows. Median age at APBSCT was 62 years (range: 38-74). Prior to transplant 8.5%, 67.4% & 24.1% were in complete, very good partial and partial remission respectively. Twenty two patients received pegfilgrastim, 84 received lenograstim from day+7 whereas some of the remaining 34 patients received varying duration of conventional G-CSF if they had no neutrophil engraftment by day +12.
Results:
Median duration for neutrophil engraftment in the pegfilgrastim, lenograstim and no G-CSF cohort was 12, 12.7 and 14 days respectively (Mann Whitney test; p value: 0.0005). Median duration for platelet engraftment in the pegfilgrastim and the no G-CSF cohort was 21.5 and 16.5 days respectively (Mann Whitney test; p value: 0.253). Median inpatient stay in the pegfilgrastim, lenograstim and the no G-CST cohort was 16, 16.5 and 18 days respectively (Mann Whitney test; p value: 0.024).
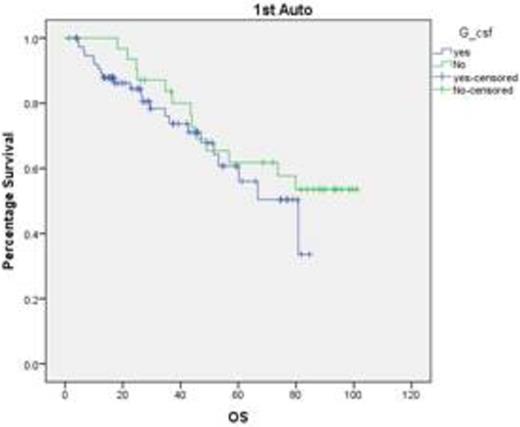
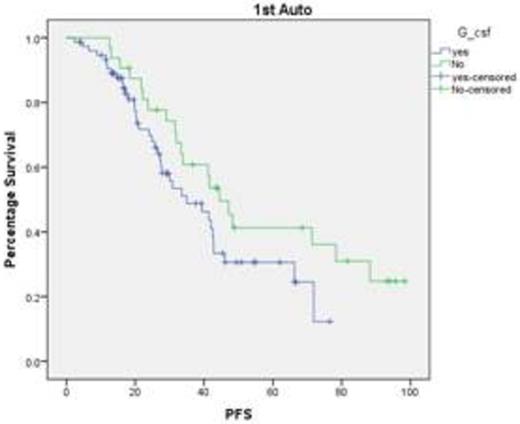
Our data suggests that the use of colony stimulating factors (pegfilgrastim & lenograstim) is associated with shorter duration to neutrophil engraftment and reduced inpatient stay which unfortunately does not translate into PFS or OS advantage. This is very useful considering the severe pressure on the bed availability in the tertiary referral centres. We also need to study whether bio similar colony stimulating factors provide the similar benefit.
Kishore:Jazz pharma: Honoraria; Celgene: Honoraria. Nikolousis:Alexion: Honoraria. Paneesha:Janssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.