Abstract
Natural killer (NK) cells are components of the innate immunity and play an important role in cancer surveillance through their cytolytic and immunomodulatory capabilities. Infusion of NK cells is a promising tool for cell therapy of hematologic malignancies and solid tumors. However, the potent cytotoxicity of NK cells might be hampered by tumor immune escape mechanisms and intrinsic resistance. We and others previously demonstrated intrinsic resistance of leukemia cells to NK cell lysis can be overcome by the transduction of artificial antigen receptor into NK cells. The genetic engineering of primary NK cells with chimeric antigen receptor improved cytotoxic activity and cytokine production, and this enhanced function was target-specific. Thus, a novel method to enhance NK cell activity against a wide range of tumors is also required. Several cytokines are associated with enhanced cytotoxicity, in vivo survival, and proliferation of NK cells. In particular, interleukin (IL)-21, which shares the common cytokine-receptor gamma chain with IL-2, was reported to enhance the cytotoxicity of human NK cells. In the present study, we investigated whether the enforced expression of human IL-21 in primary human NK cells enhanced their cytotoxicity against leukemic cells and allowed prolonged survival.
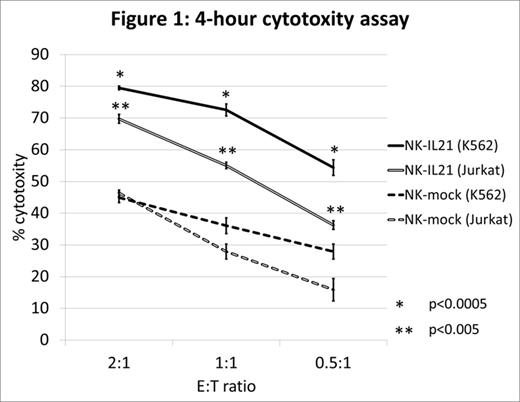
We collected peripheral blood samples from healthy adult donors, and mononuclear cells were isolated by density gradient centrifugation. Primary NK cells were expanded by stimulation with K562-mbIL15-41BBL cell line following standard procedures. After 7 days of expansion, residual T cells were removed with magnetic beads and NK cells were transduced with a retroviral vector containing human IL-21 cDNA and GFP. Fourteen days after transduction, more than 95% of cells were CD56+CD3- NK cells. Median GFP expression in the CD56+CD3- cells was 84.2% (74.5%-97.1%, n=6). We confirmed that NK cells transduced with human IL-21 cDNA (NK-IL21) had intracellular expression of IL-21 as assessed by flow cytometry, while NK cells transduced with a vector containing GFP only (NK-mock) did not. 4-hour cytotoxicity assays revealed significantly enhanced cytotoxicity exerted by NK-IL21 (Fig. 1). Cytotoxic activity of NK-IL21 against K562 cells and Jurkat cells was significantly higher than that of NK-mock. We found that the intracellular expression levels of both perforin and granzyme B were higher in NK-IL21 cells than in NK-mock cells, in accordance with their higher cytotoxicity against target cells. However, NK-IL21 did not show increased expression of the apoptosis-inducing molecule TRAIL, NK cell activating receptor NKG2D, or natural cytotoxicity receptors p30, p44, or p46.
The success of NK cell infusions might rely on the in vivo persistence of NK cells. We therefore tested whether the enforced expression of IL-21 in NK cells enhanced their proliferation and survival, and found that IL-21 expression in NK cells did not prevent apoptosis induced by IL-2 withdrawal and therefore did not favorably alter cell proliferation without IL-2. In contrast to the favorable results obtained by short-time cytotoxicity assays, NK-IL21 did not exert effective tumor control in long-term coculture experiments. The residual leukemic cell burden in NK-IL21 cocultures was not decreased and did not differ from that in NK-mock coculture experiments where cocultures were extended to 7 days without IL-2. However, by adding IL-2 (100 U/ml) to the culture, we demonstrated a dramatic suppression of residual leukemia burden exerted by NK-IL21. As shown in Figure 2, the number of residual K562 cells in the NK-IL21 cocultures was much lower than in the NK-mock cocultures (1.9% ± 0.4% vs 61.5% ± 3.8% of control culture without NK cells at a 1:1 E:T ratio, p<0.001).
These results suggest that the enforced expression of IL-21 in primary human NK cells can enhance anti-leukemic effector functions via perforin/granzyme cell death pathways, although favorable effects on NK cell proliferation and anti-apoptosis was not observed. Together with our previous observation that IL-2 gene transduction in NK cells can induce both highly enhanced proliferation and prevention of cytokine-withdrawal-induced apoptosis and activation-induced apoptosis, the transduction of both IL-2 and IL-21 genes into primary NK cells is expected to act synergistically.
Imai:Juno Therapeutics: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.