Abstract
ALK-positive Anaplastic Large Cell Lymphoma (ALCL) is a subset of Non-Hodgkin Lymphoma that positively took advantage of crizotinib development, a selective ALK inhibitor. Crizotinib is able to block ALK kinase activity which is fundamental for cancer cell survival. Constitutively active ALK kinase triggers the activation of several pro-proliferative and pro-survival downstream pathways, such as PI3K/AKT/mTOR.
Despite the positive impact of targeted therapy on ALCL treatment, resistant clones tend to be selected during therapy. Strategies to overcome resistance include the design of more potent and selective drugs and the use of combined therapy approaches that allow for the simultaneous targeting of more than one node essential for cancer cells survival. Following this strategy, we decided to investigate the effects of combined ALK/mTOR inhibition.
We first observed a synergistic effect of the combination of ALK inhibitors (crizotinib, alectinib or PF-3922) with an mTOR inhibitor (temsirolimus) in proliferation assays. Interestingly, the synergistic effect was observed only in ALK+ cell lines (Karpas 299, SUDH-L1 and SUPM2), while no synergism was observed in ALK- cell line (U937) as well as in normal lymphocytes. The Combination Index (CI) ranged from 0.16 to 0.45 indicating a synergistic/strong synergistic effect, accordingly to Chou classification. (Chou, 2006) (Table 1).
The positive cooperation resulted in an increased inhibition of mTOR effectors p70S6K, 4EBP1 and eIF4B compared to single agent treatment as observed in immunoblot analysis performed on Karpas 299 treated for 4 hours. At the cell cycle level, the use of the drugs in combination induced a sharp block in G0/G1 phase, as observed by propidium iodide analysis performed on Karpas 299 at 48-72 and 96 hours. Long term exposure of ALK+ cells to either alectinib or temsirolimus, led to the establishment of resistant cell lines, while the exposure to the combination prevented the selection of resistant clones. Interestingly, the cell line resistant to alectinib showed a marked increase of ALK both at mRNA and protein level.
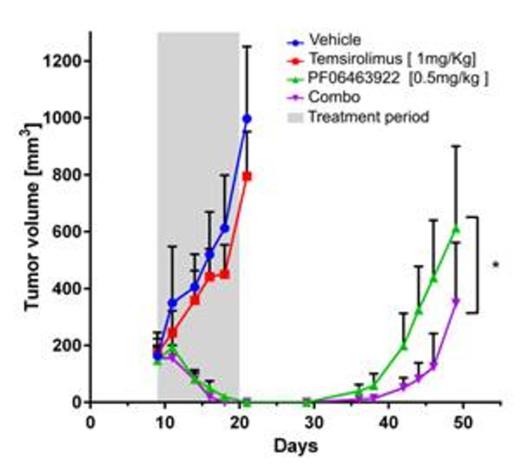
In vivo, nude mice were injected with Karpas 299 cells. As the tumor masses reached 150mm3, mice were randomized and treated for 12 days with the combination of PF-3922 (0.5 mg/kg, administered per os twice a day) and temsirolimus (i.p., 1 mg/kg every other day) or with the single agents. The combined treatment induced a faster regression of the tumor masses compared to the single agents-treated mice. Moreover, responses were sustained for a longer period of time, upon treatment stop. (Figure 1)
In conclusion, our data suggest that the combination of ALK and mTOR inhibitors could be a valuable therapeutic option for patients affected by ALK+ ALCL.
combination indexes from proliferation experiments. Synergism levels calculated accordingly to Chou, Pharmacological reviews, 2006
| . | Crizotinib - Temsirolimus . | ||||||
|---|---|---|---|---|---|---|---|
| Cell lines | Ratio | Combination Index (CI) | Average CI | Synergism level | |||
| EC50 | EC75 | EC90 | |||||
| NPM-ALK+ | Karpas 299 | 1:1 | 0.42 | 0.35 | 0.33 | 0.37 | Synergism |
| SUDHL1 | 3:1 | 0.49 | 0.45 | 0.42 | 0.45 | Synergism | |
| SUPM2 | 1:1 | 0.59 | 0.33 | 0.29 | 0.40 | Synergism | |
| NPM-ALK- | U937 | 1:1 | >10 | >10 | >10 | >10 | Antagonism |
| HD Lymphocytes | 1:1 | >10 | >10 | >10 | >10 | Antagonism | |
| Alectinib - Temsirolimus | |||||||
| Cell lines | Ratio | Combination Index (CI) | Average CI | Synergism level | |||
| EC50 | EC75 | EC90 | |||||
| NPM-ALK+ | Karpas 299 | 1:3 | 0.56 | 0.29 | 0.15 | 0.33 | Synergism |
| SUDHL1 | 1:3 | 0.53 | 0.45 | 0.38 | 0.45 | Synergism | |
| SUPM2 | 1:10 | 0.65 | 0.18 | 0.06 | 0.3 | Strong synergism | |
| NPM-ALK- | U937 | 1:3 | 1.5 | >10 | >10 | >10 | Antagonism |
| HD Lymphocytes | 1:3 | 1.2 | 2.7 | 7.2 | 3.7 | Antagonism | |
| PF06463922 - Temsirolimus | |||||||
| Cell lines | Ratio | Combination Index (CI) | Average CI | Synergism level | |||
| EC50 | EC75 | EC90 | |||||
| NPM-ALK+ | Karpas 299 | 1:10 | 0.30 | 0.15 | 0.15 | 0.20 | Strong synergism |
| SUDHL1 | 1:30 | 0.57 | 0.41 | 0.29 | 0.42 | Synergism | |
| SUPM2 | 1:3 | 0.30 | 0.12 | 0.06 | 0.16 | Strong synergism | |
| NPM-ALK- | U937 | 1:10 | 3.2 | 5.39 | >10 | >10 | Antagonism |
| HD Lymphocytes | 1:3 | 1.4 | 8.8 | >10 | >10 | Antagonism | |
| . | Crizotinib - Temsirolimus . | ||||||
|---|---|---|---|---|---|---|---|
| Cell lines | Ratio | Combination Index (CI) | Average CI | Synergism level | |||
| EC50 | EC75 | EC90 | |||||
| NPM-ALK+ | Karpas 299 | 1:1 | 0.42 | 0.35 | 0.33 | 0.37 | Synergism |
| SUDHL1 | 3:1 | 0.49 | 0.45 | 0.42 | 0.45 | Synergism | |
| SUPM2 | 1:1 | 0.59 | 0.33 | 0.29 | 0.40 | Synergism | |
| NPM-ALK- | U937 | 1:1 | >10 | >10 | >10 | >10 | Antagonism |
| HD Lymphocytes | 1:1 | >10 | >10 | >10 | >10 | Antagonism | |
| Alectinib - Temsirolimus | |||||||
| Cell lines | Ratio | Combination Index (CI) | Average CI | Synergism level | |||
| EC50 | EC75 | EC90 | |||||
| NPM-ALK+ | Karpas 299 | 1:3 | 0.56 | 0.29 | 0.15 | 0.33 | Synergism |
| SUDHL1 | 1:3 | 0.53 | 0.45 | 0.38 | 0.45 | Synergism | |
| SUPM2 | 1:10 | 0.65 | 0.18 | 0.06 | 0.3 | Strong synergism | |
| NPM-ALK- | U937 | 1:3 | 1.5 | >10 | >10 | >10 | Antagonism |
| HD Lymphocytes | 1:3 | 1.2 | 2.7 | 7.2 | 3.7 | Antagonism | |
| PF06463922 - Temsirolimus | |||||||
| Cell lines | Ratio | Combination Index (CI) | Average CI | Synergism level | |||
| EC50 | EC75 | EC90 | |||||
| NPM-ALK+ | Karpas 299 | 1:10 | 0.30 | 0.15 | 0.15 | 0.20 | Strong synergism |
| SUDHL1 | 1:30 | 0.57 | 0.41 | 0.29 | 0.42 | Synergism | |
| SUPM2 | 1:3 | 0.30 | 0.12 | 0.06 | 0.16 | Strong synergism | |
| NPM-ALK- | U937 | 1:10 | 3.2 | 5.39 | >10 | >10 | Antagonism |
| HD Lymphocytes | 1:3 | 1.4 | 8.8 | >10 | >10 | Antagonism | |
in vivo experiment for the evaluation of the combines treatment. Tumors volume measurements ±SEM are presented
in vivo experiment for the evaluation of the combines treatment. Tumors volume measurements ±SEM are presented
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.