Abstract
In recent years, engraftment syndrome (ES) has been more clearly defined in the autologous transplant setting. It represents a group of symptoms that includes fever, diarrhea, rash, pulmonary infiltrates and hepatic dysfunction, and has been associated with increased morbidity and longer hospitalization. It has also been proposed that ES may be protective against relapse.
This study cohort includes 146 patients who received 181 autologous peripheral blood stem cell transplants (PBSCT) for multiple myeloma between January 2010 and December 2014. Thirty five patients received tandem PBSCT, including 1 patient who had 3 PBSCT. Of the patients in the cohort, 63% were male, median age was 59.7 years (range 53.8 - 66.9 years) and median follow-up was 24.1 months (range 0.5 - 64.5 months). Standard definition for ES was used: at least 2 symptoms not attributed to other causes including non-infectious fever >100.4, diarrhea, skin rash, pulmonary infiltrates or hepatic dysfunction, occurring from 3 days prior to 10 days post engraftment. There were 63 occurrences of ES by defined criteria. Seven patients did not require treatment, 56 patients were treated with corticosteroids, and 2 patients required the addition of tacrolimus. Potential risk factors for the development of ES were analyzed including age, stage of disease, high-risk cytogenetics or FISH, number and type of prior regimens, mobilization regimen, number of CD34 positive cells infused, and the disease status at the time of PBSCT.
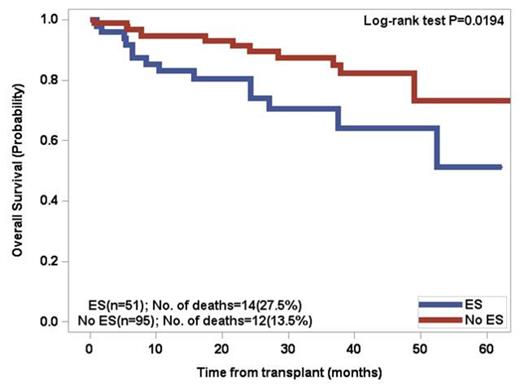
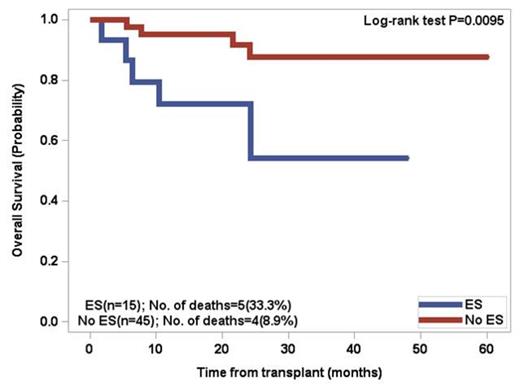
Patients above the age of 60 had a 66.7% incidence of ES whereas patientsunder the age of 60 had a 49.1% incidence. By univariate analysis, significant factors included age > 60 (p=0.037), lower number of CD34 positive cells infused (p=0.0031), and greater than 3 prior regimens excluding mobilization (p=0.0174). Two separate multivariate analyses revealed that developing ES was significantly associated with age > 60 (p=0.0372) and lower number of CD34 positive cells infused (p=0.0076). There was a trend (p=0.11) for increased incidence of ES in patients who did not receive cyclophosphamide for stem cell mobilization. For patients who developed ES, the OS at 5 years was 51.2% versus 73.1% for patients without ES (p=0.0194). Patients under the age of 60 with ES had a significant decrease in overall survival (p=0.0095) .The median progression free survival (PFS) in the overall cohort was 32.5 months, with no significant difference between the two groups.
Advanced age and lower number of CD34 positive cells infused were associated with an increased risk for the development of ES, and there was a trend for the use of cyclophosphamide for mobilization as being protective against ES. Development of ES was significantly associated with an increase in all-cause mortality. There appears to be no protective benefit of ES against relapse. Awareness that the elderly population of myeloma patients receiving autologous stem cell transplantation is more susceptible to the development of ES may help in early diagnosis and treatment. Preventative measures such as cell dose and the use of cyclophosphamide warrant further evaluation.
Overall Survival of patients <60 years old post PBSCT for multiple myeloma
Siegel:Celgene Corporation: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Novartis: Speakers Bureau; Merck: Speakers Bureau. Vesole:Celgene Corporation: Speakers Bureau; Idera Pharmaceuticals: Research Funding. Skarbnik:Genentech: Honoraria, Speakers Bureau; Gilead Sciences: Honoraria, Speakers Bureau; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.