Abstract
Background: FLT3 ITD mutated acute myeloid leukemia remains a therapeutic challenge with very few patients experiencing prolonged disease free survival after standard therapies. FLT3 inhibitors have thus far demonstrated limited single agent activity in this disease. However, administration of FLT3 inhibitors during a state of minimal residual disease and a reconstituting immune system offers a promise of improved disease control.
Methods: We designed a prospective trial for patients FLT3 ITD AML undergoing allogeneic transplant, to evaluate the safety, tolerability and outcome of the FLT3 ITD inhibitor sorafenib administered pre- and post-allogeneic transplant. To be eligible, patients needed to be in remission prior to transplant. At the discretion of the treating physician, sorafenib could be started prior to transplant after confirmation of remission and continued until three days prior to the start of the preparative regimen. Otherwise, all patients were started on sorafenib 200 mg twice a day, no earlier than Day +30 and no later than Day +120 post-transplant, after documented engraftment (Plts >50K, ANC >500). Dosing of sorafenib was based on individual patient tolerance: in tolerant patients sorafenib was escalated to 400 mg twice a day while dose reductions occurred for Grade 3 or 4 toxicities in a stepwise manner (200 mg BID, 200 mg QD, 200 mg QoD) until a tolerable dose was identified.
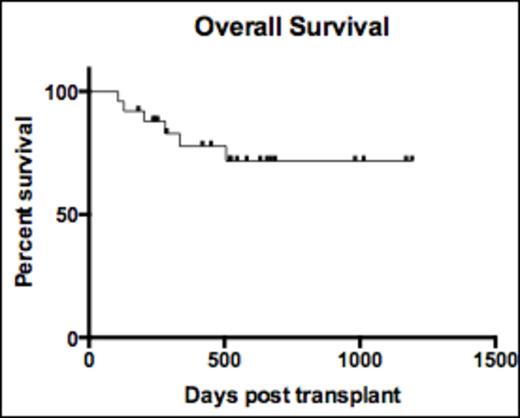
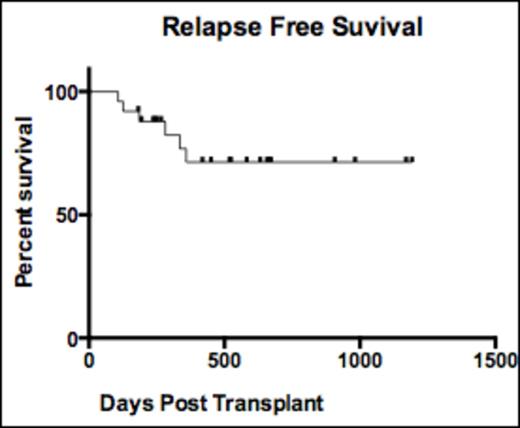
Results: We have accrued to date 28 patients with a median age of 54, with FLT ITD AML undergoing allogeneic transplant who were started on sorafenib in the peri-transplant period (8 pre-transplant). Ten patients underwent myeloablative conditioning and received a transplant from a matched related donor (5), a matched unrelated donor (2) or a haploidentical donor (3). Eighteen patients underwent non-myeloablative conditioning and transplant using either a haploidentical donor (10), a matched sibling donor (4), a matched unrelated donor (3) or an umbilical cord blood donor (1). The median duration of post transplant follow up for active patients is 450 days (range, 107-1192 days). The median duration of sorafenib therapy for all patients is 252 days (range, 52-1081). The tolerability of sorafenib in the peri-transplant period varied among patients: 6 patients tolerated the maximum dose of sorafenib at 400 mg twice a day, 14 patients were maintained on 200 mg twice a day, seven patients were maintained on 200 mg daily and one patient was maintained on 200 mg every other day. Six patients died in the post-transplant period with three deaths due to relapsed leukemia and three from transplant-associated toxicity. No primary or secondary graft failures were observed but 9 patients developed grade 2 or higher GVHD (1-4 weeks after starting sorafenib) requiring escalation of immunosuppression therapy. Overall 5 pts have relapsed so far. Three of these patients were off therapy at the time of relapse. One patient relapsed with FLT3 ITD leukemia 117 days after stopping sorafenib at the 24 month planned stoppage time and is now back in remission after resuming sorafenib.
Correlative studies evaluating FLT3 inhibition via a plasma inhibitory activity (PIA) assay showed consistent inhibition of FLT3 at all tolerability-determined dosing levels, suggesting that dosing can be safely adjusted in patients having adverse events without loss of FLT3 inhibition. Consistent with this finding was the fact that the adjusted sorafenib trough concentration exposure accounting for the active metabolite (sorafenib + (14.59*sorafenib N-oxide)) was highly variable (70% CV) and not statistically different across all dosing levels. Exploratory analysis of minimal residual disease monitoring, and of T regulatory cell populations and their relation to GVHD in the pre- and post-treatment peripheral blood, are ongoing.
Conclusion: Sorafenib is well tolerated in the post-transplant setting irrespective of the conditioning intensity or the donor source. Our findings indicate that sorafenib dosing should be individualized in the post-transplantation setting according to patient tolerability. This approach results in effective in vivo FLT3 inhibition, and yields encouraging survival results, with 15 of 28 patients currently on therapy without relapse. This data supports the further study of sorafenib in a larger cohort to determine its overall effectiveness in preventing FLT3 ITD AML relapse post allogeneic transplant.
Off Label Use: Sorafenib for off label usage in Acute Myeloid Leukemia.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract