Abstract
T stem cell memory (Tscm) represents a subset of T cells whose phenotype sits between those conventionally thought of as naive and those designated central memory. Functionally, they exhibit the capacity for self-renewal and can generate other memory cell subsets. As such, Tscm cells replenish and support the memory cell pool during infections, and contribute to immune reconstitution in patients following hematopoietic cell transplantation (HCT). Their fate and biological role in Graft-Versus-Host Disease (GVHD) is not yet understood. To address this question, we determined the dynamics and tissue-specific homeostasis of Tscm following allogeneic HCT in the translational non-human primate model of GVHD.
In this study, we tracked Tscm cells by flow cytometry at baseline, and longitudinally after MHC haploidentical HCT. Recipients were divided into 2 large groups based on clinical outcome: "Primary GVHD": Animals receiving subtherapeutic GVHD prophylaxis (either no prophylaxis, or monotherapy with either Rapamycin or CTLA4-Ig; neither of which significantly prevented GVHD); "Breakthrough GVHD": Animals receiving combined Rapamycin/CTLA4-Ig or Tacrolimus/Metotrexate (both of which prevented primary GVHD but were eventually associated with breakthrough disease). We have previously shown that reconstituting T cells in animals with primary versus breakthrough GVHD display highly significant differences in their immunophenotype and transcriptome profiles.
We have now discovered that primary GVHD is associated with a robust expansion of Tscm cells that peaks at day 5 post HCT (Fig 1A and B) and is associated with a decrease in the number of naive T lymphocytes. The Tscm compartment subsequently exhibited a contraction that accompanied an increase in the number of differentiated memory/effector T cell subsets (not shown). In contrast, animals that initially controlled GVHD maintained a delayed and prolonged expansion of Tscm cells (Fig 1A and B) that was associated with the significant preservation of naive T lymphocyte counts. Importantly, upon development of breakthrough GVHD, recipients demonstrated a transition of Tscm cells toward effector cells (Fig 1C). At the time of necropsy, animals with both primary and breakthrough GVHD displayed similar Tscm tissue distribution profiles, with these cells residing in the peripheral blood > lymph nodes > spleen > bone marrow > colon ~ liver ~ lungs.
These data suggest that during GVHD, naive T cells transit to memory/effector lymphocytes via Tscm, and that one of the key functions of GVHD immunoprophylaxis may be to limit the rate at which naive T cells enter into the Tscm and subsequent effector T cell pool.
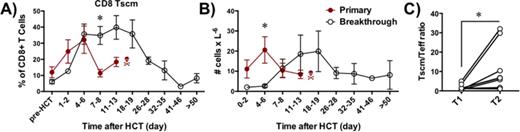
T stem cell memory dynamics during acute GVHD.
A-B) The frequencies (A) and absolute numbers (B) of CD8 T stem cell memory cells identified as CD3+CD14/20-CD8+CD45RA+CCR7+CD95+ lymphocytes and tracked longitudinally by flow cytometry following allogeneic HCT in primary (red circles) and breakthrough (black open circles) GVHD. Data display mean ± SEM. *p<0.05 using multiple t-test with Sidak-Bonferroni correction.
C) The ratio between Tscm and more differentiated memory/effector T cell subsets (Teff: summary of central memory (CD45RA-CCR7+), effector memory (CD45RA-CCR7-) and terminally differentiated effector (CD45RA+CCR7-) CD8+ T cells at 2 different time points: (1) At the time of maximal expansion of Tscm cells prior to the clinical development of breakthrough GVHD (T1) and (2) When recipients developed clinically significant breakthrough GVHD (T2). *p<0.05 using paired t-test.
T stem cell memory dynamics during acute GVHD.
A-B) The frequencies (A) and absolute numbers (B) of CD8 T stem cell memory cells identified as CD3+CD14/20-CD8+CD45RA+CCR7+CD95+ lymphocytes and tracked longitudinally by flow cytometry following allogeneic HCT in primary (red circles) and breakthrough (black open circles) GVHD. Data display mean ± SEM. *p<0.05 using multiple t-test with Sidak-Bonferroni correction.
C) The ratio between Tscm and more differentiated memory/effector T cell subsets (Teff: summary of central memory (CD45RA-CCR7+), effector memory (CD45RA-CCR7-) and terminally differentiated effector (CD45RA+CCR7-) CD8+ T cells at 2 different time points: (1) At the time of maximal expansion of Tscm cells prior to the clinical development of breakthrough GVHD (T1) and (2) When recipients developed clinically significant breakthrough GVHD (T2). *p<0.05 using paired t-test.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.