Abstract
Introduction: PTCL is a rare and heterogeneous group of non-Hodgkin lymphoma (NHL) comprising ~10% of cases. CHOP is frequently used first-line, but with the exception of ALK+ anaplastic large-cell lymphoma (ALCL), long term outcomes are historically poor with reported 5-yr overall survival (OS) rates of 36%. We retrospectively evaluated the outcomes following first-line chemotherapy for patients with PTCL treated at the Royal Marsden (RM) and Christie (CH) hospitals over a 10-year period.
Methods: All eligible patients with PTCL aged ≥18 years and treated at the RM and CH between 1st January 2002 and 31st January 2012 were included. The study was approved by our institutional review boards. Patients were identified from hospital databases and included if they had received at least 1 cycle of first-line chemotherapy. Precursor T-cell malignancies, mycosis fungoides and adult T-cell leukaemia/lymphoma were excluded, as was cutaneous T-cell lymphoma not requiring combination chemotherapy. Clinical data were collated from electronic patient records and the diagnosis of PTCL was confirmed in all cases by an expert haematopathologist. Response was assessed using the IWG 1999 criteria. OS and progression free survival (PFS) were calculated from date of start of 1st line treatment and analysed using Kaplan Meier methods and Cox regression model. The impact of clinical factors on survival was assessed using Cox regression analysis.
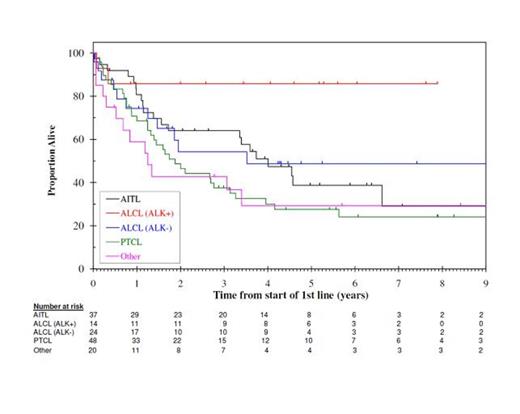
Results: A total of 143 (RM n=69, CH n=74)patients were evaluable and the median follow-up was 63.4 months. The median age at diagnosis was 59 yrs (range 18-89 yrs). PTCL subtypes were: PTCL not otherwise specified (NOS) (n=48), angioimmunoblastic T-cell lymphoma (AITL) (n=37), ALCL ALK- (n=24), ALCL ALK+ (n=14) and other (n=20). First-line chemotherapy included CHOP (n=97), GEM-P (gemcitabine, cisplatin and methylprednisolone) (n=16), other gemcitabine containing regimen (n=7), asparaginase (n=2) or other (n=21). OS by PTCL subtype is shown in Figure 1.
Response was evaluable for 125/143 patients. Overall response (ORR) to first-line chemotherapy was 81.4% with complete response (CR) seen in 42.4%. For the entire cohort (n=143) 5-yr PFS was 20.6% and 5-yr OS was 39.6%. For CHOP treated patients ORR was 80.5% with CR in 43.7%, 5-yr PFS was 25.5% and 5-yr OS was 41.2%. ORR with GEM-P was 78.6% with CR in 50%, 5-yr PFS was 13.6% and 5-yr OS was 39.1%. No statistically significant difference between CHOP and GEM-P was seen in terms of response, OS or PFS.
Autologous stem cell transplantation (autoSCT) was performed post first-line induction in 15% (n=22). For patients in CR post induction (CR1) (n=41), we compared survival for those treated with (n=12) and without (n=29) subsequent autoSCT. AutoSCT in CR1 was associated with a trend towards better PFS (HR 0.36, 95%CI 0.13-1.02; p=0.056) but not OS (HR 0.72, 95% CI 0.21-2.47; p=0.599).
Uni- (UVA) and multivariate analyses (MVA) were performed to determine the impact of the following on OS and PFS: age (≤60 vs > 60yrs), gender, stage (I-III vs IV), performance status (PS, 0-1 vs 2), B symptoms (present vs absent), ethnicity (white vs other), LDH (normal vs elevated), IPI (low vs intermediate vs high), PTCL subtype, number of extranodal sites (0-1 vs >1), chemotherapy (CHOP vs gemcitabine based vs other), CR post induction (present vs absent) and autoSCT (performed vs not). Factors with a p-value of <0.1 in UVA were entered into MVA and independently significant risk factors (p<0.05) are shown in Table 1.
Conclusion: With the exception of ALK+ ALCL, outcomes for PTCL following first-line chemotherapy remain disappointing and a more effective induction regimen is urgently required. Our data indicates that achieving a CR with first-line induction is a key factor for optimising OS and PFS. To this end, the currently accruing UK NCRI randomised phase II CHEMO-T study is comparing CHOP with GEM-P as first-line regimens in PTCL. AutoSCT in CR1 may offer a PFS benefit and should be considered in eligible patients.
MVA for OS and PFS (n=104)
| Risk Factor . | HR . | 95% CI . | p-value . |
|---|---|---|---|
| OS | |||
| Intermediate risk IPI | 4.56 | 1.79-11.6 | 0.001 |
| High risk IPI | 12.0 | 4.32-33.1 | <0.001 |
| CR post induction | 0.36 | 0.21-0.64 | <0.001 |
| PFS | |||
| Female sex | 0.33 | 0.19-0.58 | <0.001 |
| Intermediate risk IPI | 3.17 | 1.61-6.24 | 0.001 |
| High risk IPI | 7.76 | 3.35-17.6 | <0.001 |
| CR post induction | 0.36 | 0.22-0.60 | <0.001 |
| AutoSCT post induction | 0.30 | 0.13-0.66 | 0.003 |
| Risk Factor . | HR . | 95% CI . | p-value . |
|---|---|---|---|
| OS | |||
| Intermediate risk IPI | 4.56 | 1.79-11.6 | 0.001 |
| High risk IPI | 12.0 | 4.32-33.1 | <0.001 |
| CR post induction | 0.36 | 0.21-0.64 | <0.001 |
| PFS | |||
| Female sex | 0.33 | 0.19-0.58 | <0.001 |
| Intermediate risk IPI | 3.17 | 1.61-6.24 | 0.001 |
| High risk IPI | 7.76 | 3.35-17.6 | <0.001 |
| CR post induction | 0.36 | 0.22-0.60 | <0.001 |
| AutoSCT post induction | 0.30 | 0.13-0.66 | 0.003 |
Chau:Roche: Research Funding. Cunningham:Merrimack: Research Funding; Bayer HealthCare Pharmaceuticals: Research Funding; Medimmune: Research Funding; Astra Zeneca: Research Funding; Amgen: Research Funding; Celgene: Research Funding; Merck Serono: Research Funding; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.