To the editor:
Indeterminate cell histiocytosis (ICH) is a rare and controversial disorder first described by Wood et al1 in 1985. ICH is characterized by a nonepidermotropic histiocytic infiltrate with immunohistochemical features that overlap with Langerhans cells (LCs) and non-LCs of monocyte-macrophage-dendritic cell lineage.1 It is unclear whether ICH is distinct from Langerhans cell histiocytosis (LCH) or instead represents a variant form of LCH. The gold standard for distinguishing ICH from LCH is demonstration of the absence of Birbeck granules by electron microscopy or, more practically, through use of langerin (CD207) immunohistochemistry (IHC), which is positive in LCH and non-reactive in ICH.2 Although LCH is understood to be a clonal proliferation, there has not been sufficient support for a common molecular pathway in ICH in the literature to date, leading some authors to consider ICH a reactive condition. Herein, we present 3 cases of ICH with a recurrent ETV3-NCOA2 translocation. This molecular finding provides evidence that ICH is a true clonal entity deserving of separate classification.
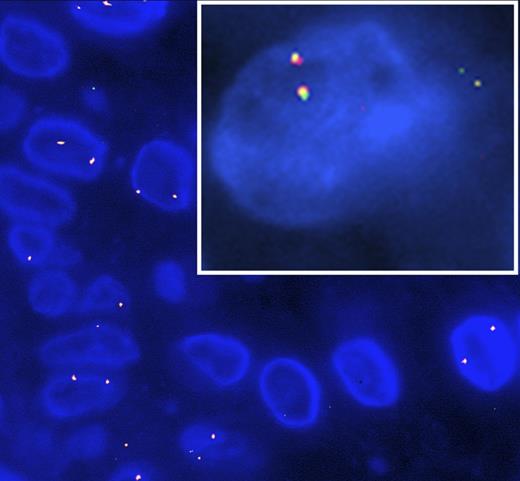
Patient 1 was a 62-year-old woman who presented with a 30-year history of innumerable dome-shaped smooth red and brown papules and nodules scattered diffusely over her face, chest, back, and extremities. Skin biopsy demonstrated a dermal infiltrate of mononuclear histiocytes highlighted by CD1a and CD68 without langerin reactivity. Scattered mature lymphocytes and rare eosinophils were intermixed. S100 was positive in 5% of the histiocytic infiltrate. Electron microscopy confirmed the absence of Birbeck granules. Targeted next-generation sequencing was performed by Foundation Medicine (Cambridge, MA) using the previously described FoundationOne assay,3 and it revealed an ETV3-NCOA2 gene fusion characterized as 5′-ETV3(x1-4)+NCOA2(x14-23)-3′ resulting in an aberrant NCOA2. Other aberrant mutations, including those involving the RAS-RAF-MEK pathway, were not found. BRAF V600E IHC was negative. Fluorescence in situ hybridization (FISH) for the ETV3-NCOA2 translocation was positive (Figure 1, inset).
FISH targeting the ETV3-NCOA2 gene fusion in ICH. Red probe for ETV3 in chromosome 1 and green probe for NCOA2 in chromosome 8. ETV3-NCOA2 gene fusion (yellow) present in paraffin embedded tissue taken from patient 2 and patient 1 (inset).
FISH targeting the ETV3-NCOA2 gene fusion in ICH. Red probe for ETV3 in chromosome 1 and green probe for NCOA2 in chromosome 8. ETV3-NCOA2 gene fusion (yellow) present in paraffin embedded tissue taken from patient 2 and patient 1 (inset).
Patient 2 was a 51-year-old woman who presented with a several-week history of diffuse nonpruritic papules involving her arms, legs, neck, scalp, face, chest, and back. Histopathologic examination of a skin biopsy demonstrated a dermal infiltrate composed of mononuclear histiocytes with eosinophilic cytoplasm and admixed mature-appearing lymphocytes. The histiocytes were positive for S100, CD68, and CD1a, whereas langerin and BRAF V600E IHC were negative. FISH testing for the ETV3-NCOA2 translocation was positive (Figure 1).
Patient 3 was an 81-year-old man who presented with a solitary lesion on the upper arm which had been present for approximately 6 months. Biopsy showed a multinodular dermal infiltrate of large, oval cells with abundant pale eosinophilic cytoplasm and reniform nuclei. The lesional cells were positive for S100, CD68, and CD1a, whereas langerin and BRAF V600E IHC were negative. FISH testing for the ETV3-NCOA2 translocation was positive.
A fourth patient with ICH was screened with ETV3-NCOA2 FISH and was negative. FISH targeting the ETV3-NCOA2 translocation was performed on tissue from a control group of non-ICH patients (1 previously described case of congenital LCH with background indeterminate cells,4 11 cases of LCH, 4 cases of juvenile xanthogranuloma, and 1 case of Rosai-Dorfman disease), all of which were negative. FISH studies were performed with positive and negative metaphase controls demonstrating appropriate results. All cases expressed CD68 by IHC and showed a histiocyte-predominate infiltrate histopathologically. All cases of LCH were reactive for langerin and CD1a, whereas all cases that were neither ICH nor LCH were negative for these markers. Three of 5 LCH cases tested with BRAF V600E IHC were positive, as is consistent with the literature.5 The Rosai-Dorfman disease case expressed S100 by IHC and demonstrated emperipolesis by conventional microscopy. The juvenile xanthogranuloma cases were S100 negative and included Touton-type multinucleated cells.
Herein, we present 3 cases of ICH with an ETV3-NCOA2 translocation, which appears absent in other histiocytic disorders, most importantly LCH. Furthermore, the case of LCH with an increased population of indeterminate cells fails to demonstrate this translocation, indicating that ICH may represent a clonal disorder that is distinct from reactive populations of indeterminate cells. LCH is understood to represent a clonal proliferation of LCs, with recent studies demonstrating alterations in the RAS-RAF-MEK pathway, most notably a BRAF V600E mutation in 50% to 60% of patients.5 In contrast, strong support for ICH clonality has not been presented to date. NCOA2 encodes nuclear receptor coactivator 2, a transcription factor required for homeostatic balance between white and brown adipose tissue.6 ETV3 encodes Ets variant 3, a transcriptional repressor involved in macrophage growth arrest via inhibition of Ras-dependent proliferation.7 Translocations involving NCOA2 have been observed in mesenchymal chondrosarcoma, spindle cell rhabdomyosarcoma, prostate cancer, colon cancer, acute leukemia, and soft tissue angiofibroma, whereas ETV3 abnormalities have been noted in brain and breast cancer.6-9 Neoplastic associations of ICH are limited to case reports that include patients with mast cell leukemia, acute myeloid leukemia, and B-cell lymphoma.10 Patient 2 had a remote history of breast cancer treated with chemotherapy; however, no patient was noted to have a malignancy at the time of their ICH diagnosis or at 5 and 4 months (patients 1 and 2, respectively) after diagnosis based on available follow-up information. The ETV3-NCOA2 gene fusion presents a potential diagnostic and therapeutic tool for addressing the challenges associated with the controversial and rare entity that is ICH and merits further exploration.
Authorship
Contribution: R.A.B. collected cases and wrote the paper; B.Y.K., B.R., and T.H.M. provided clinical information and case material; L.M. and C.C. performed research; K.E.R. provided case material; D.A.A. provided case material and designed research; and J.K. designed research, analyzed data, provided case material, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jinah Kim, Dermatopathology Service, Stanford University School of Medicine, 300 Pasteur Drive, L235, Stanford, CA 94305; e-mail: jinahkim@stanford.edu.