Key Points
In SCD, recurrent vaso-occlusive crisis suppresses osteogenic lineage and activates osteoclasts.
Zoledronic acid acting on both osteoclast and osteoblast compartments is a multimodal therapy to prevent SBD.
Abstract
Sickle cell disease (SCD) is a worldwide distributed hereditary red cell disorder, characterized by severe organ complication. Sickle bone disease (SBD) affects a large part of the SCD patient population, and its pathogenesis has been only partially investigated. Here, we studied bone homeostasis in a humanized mouse model for SCD. Under normoxia, SCD mice display bone loss and bone impairment, with increased osteoclast and reduced osteoblast activity. Hypoxia/reperfusion (H/R) stress, mimicking acute vaso-occlusive crises (VOCs), increased bone turnover, osteoclast activity (RankL), and osteoclast recruitment (Rank) with upregulation of IL-6 as proresorptive cytokine. This was associated with further suppression of osteogenic lineage (Runx2, Sparc). To interfere with the development of SBD, zoledronic acid (Zol), a potent inhibitor of osteoclast activity/osteoclastogenesis and promoter of osteogenic lineage, was used in H/R-exposed mice. Zol markedly inhibited osteoclast activity and recruitment, promoting osteogenic lineage. The recurrent H/R stress further worsened bone structure, increased bone turnover, depressed osteoblastogenesis (Runx2, Sparc), and increased both osteoclast activity (RankL, Cathepsin k) and osteoclast recruitment (Rank) in SCD mice compared with either normoxic or single-H/R-episode SCD mice. Zol used before recurrent VOCs prevented bone impairment and promoted osteogenic lineage. Our findings support the view that SBD is related to osteoblast impairment, and increased osteoclast activity resulted from local hypoxia, oxidative stress, and the release of proresorptive cytokine such as IL-6. Zol might act on both the osteoclast and osteoblast compartments as multimodal therapy to prevent SBD.
Introduction
Sickle cell disease (SCD) is a worldwide distributed hereditary red cell disorder, which affects approximately 75 000 individuals in the United States and almost 20 000 to 25 000 individuals in Europe, this latter mainly related to the immigration fluxes from endemic areas such as Sub-Saharan Africa to European countries.1-3 Studies of the global burden of disease have pointed out the invalidating impact of SCD on patient quality of life.2 This requires the development of new therapeutic options to treat sickle cell–related acute and chronic complications.
SCD is caused by a point mutation in the β-globin gene resulting in the synthesis of pathologic hemoglobin S (HbS). HbS displays peculiar biochemical characteristics, polymerizing when deoxygenated with associated reduction in cell ion and water content (cell dehydration), increased red cell density, and further acceleration of HbS polymerization.4-6 Pathophysiologic studies have shown that dense, dehydrated red cells play a central role in acute and chronic clinical manifestations of SCD, in which intravascular sickling in capillaries and small vessels leads to vaso-occlusion and impaired blood flow with ischemic/reperfusion injury.4,7-9 In microcirculation, vaso-occlusive events (VOCs) result from a complex and still only partially known scenario involving the interactions between different cell types, including dense red cells, reticulocytes, abnormally activated endothelial cells, leukocytes, platelets, and plasma factors.4,9,10
Target organs, such as bone or lung, are involved in both acute and chronic clinical manifestations of SCD, related to their peculiar anatomic organization mainly characterized by sluggish circulation and relative local hypoxia.11 Although progress has been made in the knowledge of sickle cell–related organ damage, much still remains to be investigated in the pathogenesis of sickle bone disease (SBD).12-14 Bone imaging studies by magnetic resonance imaging of SCD patients in the early phase of VOCs have documented the presence of abnormal periosteal signal intensity, which then develops in areas of bone infarctions.12 Thus, VOCs combined with marrow hyperplasia and inflammation have been suggested to contribute to the development of SBD.12,13 Recently, a possible role of vitamin D deficiency in SBD has been proposed, which appears to be subordinated to the primary defect in bone homeostasis.12
Studies in other models have shown that bone remodeling is a complex process, involving osteoblastic and osteoclastic activity and promoting bone formation/resorption. To this process, the transcription factor Runx2 plays a pivotal role for osteoblastic differentiation by activating downstream genes such as Sparc (osteonectin). However, the osteoclastic differentiation and activation is related to osteoclast cell–surface receptor, Rank.15,16
Here we demonstrate that SBD results from the combined reduced osteoblast recruitment and increased osteoclast activity. Recurrent hypoxia/reoxygenation (H/R) events, mimicking acute VOCs, activate osteoclastogenesis and bone turnover. In SCD mice, zoledronic acid (Zol) ameliorates bone impairment by restraining osteoclast activity in favor of osteoblasts. In a model of recurrent VOCs, Zol acts on both the osteoclast and osteoblast compartments as multimodal therapy, preventing bone loss and bone impairment. Our study is the first to report on osteoblasts in SBD and provides the rationale of Zol as therapeutic intervention to prevent SBD.
Materials and methods
Mouse model and study design
Experiments were performed on healthy control (Hbatm1(HBA)TowHbbtm3(HBG1,HBB)Tow AA) and SCD (Hbatm1(HBA)TowHbbtm2(HBG1,HBB*)Tow SS) mice.8,17 The Institutional Animal Experimental Committee, University of Verona (CIRSAL), and the Italian Ministry of Health approved the experimental protocols. Sex- (M:F ratio 1:1) and age- (16-18 weeks) matched mice were studied.18 Whenever indicated, mice were exposed to hypoxia (8% oxygen for 10 hours) followed by 3 hours reoxygenation (21% oxygen) (H/R stress) to mimic a single acute VOC as previously described.17,19,20 For recurrent VOCs, AA and SS mice were exposed to 3 times VOC (8% oxygen for 10 hours followed by 3 hours reoxygenation on 21% oxygen) with a 3-week interval between each VOC.
Hematologic parameters, red cell indices, and reticulocyte counts were determined as previously reported.21,22 Blood was collected with retro-orbital venipuncture in anesthetized mice using heparin microcapillary tubes. Hematologic parameters were evaluated on a Technicon Analyzer ADVIA (Bayer). Hematocrit and hemoglobin measurements were determined manually. Whenever indicated, Zol was administrated at a dosage of 100 μg/kg intraperitoneally (IP) based on previous reports.23,24
Flow cytometric analysis of mouse erythroid precursors of murine bone marrow erythroblasts
Measurements of bone homeostasis and turnover
For details of bone histomorphometry and bone turnover, see supplemental Methods, available on the Blood Web site.27-30 The number of nodes (branch points) and termini (the end points) in a trabecular network, which was skeletonized to facilitate examination of its topologic properties, were analyzed according to Garrahan et al.31 In a bone section, the ratio of nodes to termini (Nd/Tm) is an index of spatial connectivity. Thus, higher values correspond to better connectivity.32 In bone histomorphometry, marrow star volume (MSV) is defined as the mean volume of all parts of an object that can be seen unobscured from a random point within the object.33 Higher values of MSV correspond to reduced connectivity.33 To evaluate bone resorption, the number of osteoclast/tissue area (NOc/TA) and erosion surface/bone surface (ES/BS), corresponding to bone covered by eroded cavities, were measured according to international guidelines.29
Colony-forming unit–osteoblast assay
A colony-forming unit–osteoblast (CFU-Ob) assay was conducted as previously reported.34 Briefly, 1 × 106 bone marrow nucleated cells from AA and SS mice were seeded in 60-mm culture dishes in osteogenic differentiation medium (α-minimum essential medium supplemented with 50 µg/mL l-ascorbic acid and 2.0 mM β-glycerophosphate) and incubated at 37°C under a humidified atmosphere of 5% CO2. The medium was changed every 2 days, and after 21 days of culture, CFU-Ob colonies were identified by alizarin red staining as previously described.34,35
Bone total RNA extraction and reverse transcription
Total RNA was extracted from each pellet using the RNeasy minikit (QIAGEN, Milano Italy) with DNAse I treatment. Tibia bones were harvested from mice and any attached tissue was removed before RNAlater solution (Thermo Fisher) was added and stored at 4°C. After 24 hours, the RNAlater was eliminated and the samples were stored at −80°C until RNA extraction. Frozen samples were crushed using a mortar and pestle in liquid nitrogen as previously described36 and then homogenized in RLT Lysis buffer. Details are reported in the supplemental Methods. Real-time polymerase chain reaction was performed as previously described.37 Details are reported in the supplemental Methods.
Serum bone alkaline phosphatase and CTX-I evaluation
Sera were collected and stored at −80° until analysis. The amounts of bone alkaline phosphatase (ALP) and CTX-I were determined using a mouse bone–specific alkaline phosphatase (BALP) Elisa Kit (CSB-E11914m; Cusabio) and the CTX –I ELISA kit (CSB-E12782m; Cusabio), respectively. The assays were performed on 96-well immunoplates, according to the manufacturer instructions.
Statistical analysis
Statistical analyses were performed using SPSS for Windows version 21.0 (SPSS Inc./IBM, Armonk, NY). The 2-way analysis of variance algorithm for repeated measures was used for data analysis. Differences with P < .05 were considered significant.
Results
Sickle cell mice show bone structure impairment and increases osteoclast activity
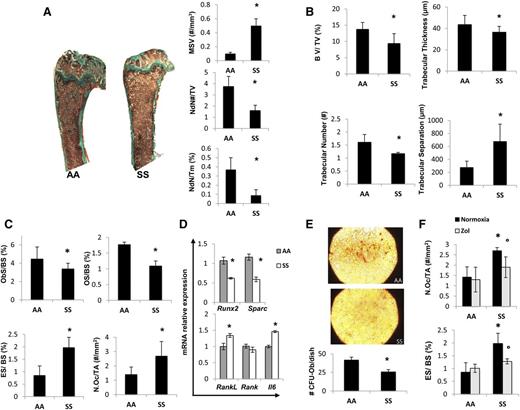
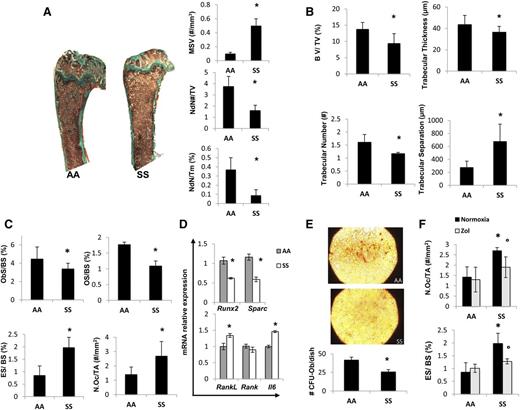
To study the pathogenesis of SBD related to the hematologic phenotype, we studied humanized SS mice fed with a standard diet containing 0.97% calcium, 0.85% phosphorus, 1045 U/kg vitamin D3, 22.5% protein, 5.5% fat, and 52% carbohydrate to avoid confounding contribution of vitamin D deficiency on bone homeostasis. SCD mice displayed reduced node number and node/termini ratio with increased MSV compared with control mice expressing normal hemoglobin (AA), suggesting an impairment of bone microarchitecture (Figure 1A). In SCD mice, we also found erythroid hyperplasia without perturbation of erythroid maturation profile compared with controls similarly to human SCD subjects and in agreement with the increased MSV documented in SCD mice (supplemental Figure 1A).
Sickle cell mice display bone impairment, increased osteoclast activity, and suppression of osteoblastogenesis. Beneficial effects of zoledronic acid (Zol) on osteoclast activity in sickle cell mice. (A) Quantitative histomorphometry of the distal femur of healthy (AA) and sickle cell (SS) mice. Upper panel: representative undecalcified section of distal femur stained with trichrome Goldner’s stain. Lower panel: MSV, marrow star volume; NdN/TV, node number/tissue volume; NdN/Tm, node to termini ratio. Data are shown as mean ± standard deviation (SD) (n = 6); *P < .05 compared with healthy mice. (B) Bone volume (BV)/tissue volume (TV), trabecular thickness, trabecular number, and trabecular separation measured by Bone Explora Nova 3.5 image analyzer. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice. (C) ObS/ BS, osteoblast surface/bone surface; OS/BS, osteoid surface/bone surface; N.Oc/TA, osteoclast number/tissue area; ES/BS, Erosion surface/bone surface. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice. (D) Real-Time PCR analysis of Runx2, Sparc, RankL, Rank, and Il6. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice. (E) To assess the number of osteoprogenitors, bone marrow–derived cells were cultured in vitro under osteogenic differentiation conditions. The CFU-Ob obtained from the MSCs of AA and SS mice were stained by alizarin red (upper panel) and quantified (#CFU-Ob/dish) (lower panel); data are shown as mean ± SD (n = 3; P < .05 AA vs SS). (F) ES/BS, erosion surface/bone surface; N.Oc/TA, osteoclast number/tissue area in AA and SS mice with and without Zol treatment (100 μg/kg IP). Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice.
Sickle cell mice display bone impairment, increased osteoclast activity, and suppression of osteoblastogenesis. Beneficial effects of zoledronic acid (Zol) on osteoclast activity in sickle cell mice. (A) Quantitative histomorphometry of the distal femur of healthy (AA) and sickle cell (SS) mice. Upper panel: representative undecalcified section of distal femur stained with trichrome Goldner’s stain. Lower panel: MSV, marrow star volume; NdN/TV, node number/tissue volume; NdN/Tm, node to termini ratio. Data are shown as mean ± standard deviation (SD) (n = 6); *P < .05 compared with healthy mice. (B) Bone volume (BV)/tissue volume (TV), trabecular thickness, trabecular number, and trabecular separation measured by Bone Explora Nova 3.5 image analyzer. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice. (C) ObS/ BS, osteoblast surface/bone surface; OS/BS, osteoid surface/bone surface; N.Oc/TA, osteoclast number/tissue area; ES/BS, Erosion surface/bone surface. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice. (D) Real-Time PCR analysis of Runx2, Sparc, RankL, Rank, and Il6. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice. (E) To assess the number of osteoprogenitors, bone marrow–derived cells were cultured in vitro under osteogenic differentiation conditions. The CFU-Ob obtained from the MSCs of AA and SS mice were stained by alizarin red (upper panel) and quantified (#CFU-Ob/dish) (lower panel); data are shown as mean ± SD (n = 3; P < .05 AA vs SS). (F) ES/BS, erosion surface/bone surface; N.Oc/TA, osteoclast number/tissue area in AA and SS mice with and without Zol treatment (100 μg/kg IP). Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice.
Bone volume was significantly decreased in SCD compared with healthy mice (Figure 1B). Trabecular width and thickness were decreased, whereas the trabecular separation was increased in SCD mice compared with controls, indicating the presence of bone loss in SCD mice (Figure 1B). This finding is in agreement with a previous preliminary report in the same mouse model.18
Because bone homeostasis is mainly related to the balance between bone resorption/bone formation, we evaluated osteoblast/osteoclast function in both mouse strains. In SCD mice, we found reduced number of osteoblasts (OBs/BS, Figure 1C; N.Ob/BS, supplemental Figure 1B) associated with decreased osteoid surface (Figure 1C), indicating a lower osteoblast activity in SCD compared with healthy mice. These findings were in agreement with the downregulation of molecular osteogenic markers such as Runx2 and Sparc (osteonectin) in SCD mice (Figure 1D). However, osteoclastic activity was markedly increased as supported by a higher osteoclast number associated with increased erosion surface in SCD mice compared with controls (Figure 1C). RankL, a marker of osteoclast activation, was significantly upregulated in SCD mice compared with healthy controls (Figure 1D), although no significant changes in the expression of Rank, a marker of osteoclast recruitment, was observed in SCD mice (Figure 1D). We also analyzed the expression of IL-6, a pro-resorptive cytokine that cooperates with RankL on osteoclast activation.38,39 As shown in Figure 1D, IL-6 expression was upregulated in SCD mice compared with controls. These data indicate increased osteoclast activity combined with possible perturbation of the osteoprogenitor compartment in SCD mice. Indeed, we found a decreased ability of mesenchymal stem cells (MSCs) from SCD mice to form osteoblast-CFUs compared with healthy controls, suggesting a defect in MSC recruitment to osteoblasts in SCD mice (Figure 1E).
Because antioxidant systems, such as peroxiredoxin-2 (Prx2) or catalase, have been recently reported to negatively regulate osteoclastogenesis, we analyzed the expression of both antioxidant systems in bones from both SCD and control mice.40,41 Prx2 gene expression was markedly upregulated in SCD mice compared with healthy controls (supplemental Figure 1C), whereas Catalase expression was similar in both mouse strains (data not shown).
No changes in bone turnover, measured by calcein double-labeling, were present in SCD mice compared with controls, which was combined with similar levels of carboxy-terminal collagen crosslinks (CTX-I) in both mouse strains (data not shown).
These data suggest that SBD is sustained by a shift in the balance between osteoblast/osteoclast activity toward osteoclasts and the upregulation of Prx2 antioxidant system as a back-up mechanism to control oxidative stress and limit osteoclast activity in SCD mice.
Zoledronic acid prevents bone loss and ameliorates osteogenic differentiation in SCD mice
Until now, Zol is considered the most powerful amino-bisphosphonate that inhibits osteoclast activity and osteoclastogenesis and promotes osteogenic differentiation.27,28 We then treated SCD and control mice with Zol (100 μg/kg IP, single dose)23 to investigate whether it might effectively counteract bone impairment of SCD mice.
Zol significantly reduced osteoclast number and erosion surface in SCD mice without major changes in healthy mice (Figure 1E). This was associated with upregulation of Runx2 and Sparc (osteonectin) and downregulation of Rank genes expression in both mouse models (supplemental Figure 2). Prx2 expression was markedly decreased in Zol-treated SCD mice compared with vehicle-treated mice (supplemental Figure 2).
Taken together, these data suggest that in SCD mice, Zol decreases osteoclastic activity and promotes osteoblastogenesis in SCD mice.
Hypoxia/reoxygenation stress mimics acute vaso-occlusive crisis and increases bone turnover in SCD mice
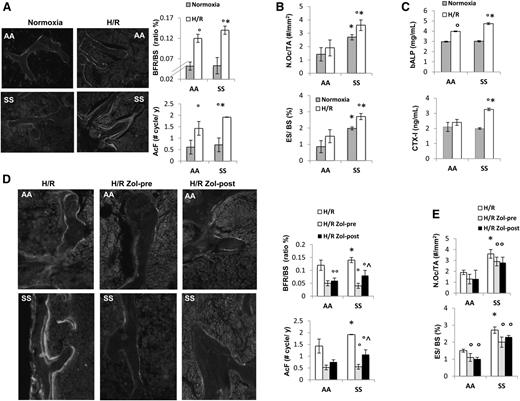
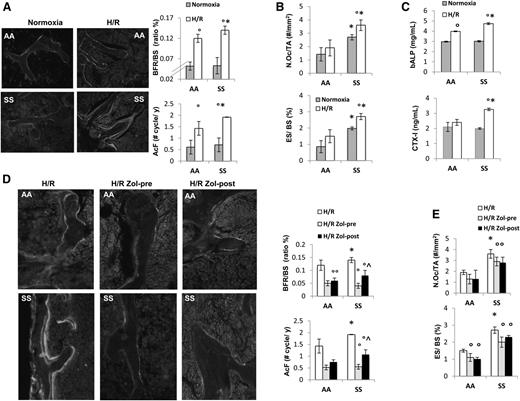
Previous studies have shown that H/R stress mimics acute VOC in different mouse models for SCD.17,21,22 Thus, we exposed SCD and healthy mice to H/R stress. As previously reported, H/R stress induced increased neutrophil count and decreased hemoglobin levels (supplemental Table 1).17 Increased bone turnover induced by H/R stress was present in both mouse groups and was higher in SCD mice compared with controls (Figure 2A and supplemental Table 2). This was associated with significant increases in osteoclast number and erosion surface in SCD mice only (Figure 2B). bALP increased in both AA and SS mice exposed to H/R, whereas CTX-I significantly increased in SCD mice only (Figure 2C).
Hypoxia/reoxygenation stress (H/R) affects bone homeostasis in sickle cell mice. Zoledronic acid prevents H/R-induced increased turnover and osteoclast activation. (A) Left panel: representative images of calcein-labeled bone surface. Right panel: bone formation rate (BFR/BS) and activation frequency (AcF) in healthy (AA) and sickle cell (SS) mice under normoxia and exposed to H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with normoxic mice. (B) N.Oc/TA, osteoclast number/tissue area; ES/BS, erosion surface/bone surface in AA and SS mice under normoxia and exposed to H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with normoxic mice. (C) Serum levels of bone alkaline phosphatase (bALP) and carboxy-terminal collagen crosslinks (CTX-I) in AA and SS mice under normoxia and exposed to H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with normoxic mice. (D) Left panel: representative images of calcein-labeled bone surface. Right panel: BRF/BS and AcF in AA and SS mice exposed to H/R stress without and with Zol treatment before (pre-Zol) or after (Zol-post) H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle treated mice; ^P < .05 Zol-pre compared with Zol-post. (E) N.Oc/TA and ES/BS in AA and SS mice exposed to H/R stress without and with Zol treatment before (Zol-pre) or after (Zol-post) H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with vehicle-treated mice.
Hypoxia/reoxygenation stress (H/R) affects bone homeostasis in sickle cell mice. Zoledronic acid prevents H/R-induced increased turnover and osteoclast activation. (A) Left panel: representative images of calcein-labeled bone surface. Right panel: bone formation rate (BFR/BS) and activation frequency (AcF) in healthy (AA) and sickle cell (SS) mice under normoxia and exposed to H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with normoxic mice. (B) N.Oc/TA, osteoclast number/tissue area; ES/BS, erosion surface/bone surface in AA and SS mice under normoxia and exposed to H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with normoxic mice. (C) Serum levels of bone alkaline phosphatase (bALP) and carboxy-terminal collagen crosslinks (CTX-I) in AA and SS mice under normoxia and exposed to H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with normoxic mice. (D) Left panel: representative images of calcein-labeled bone surface. Right panel: BRF/BS and AcF in AA and SS mice exposed to H/R stress without and with Zol treatment before (pre-Zol) or after (Zol-post) H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle treated mice; ^P < .05 Zol-pre compared with Zol-post. (E) N.Oc/TA and ES/BS in AA and SS mice exposed to H/R stress without and with Zol treatment before (Zol-pre) or after (Zol-post) H/R stress. Data are shown as mean ± SD (n = 6); *P < .05 compared with AA; °P < .05 compared with vehicle-treated mice.
In both mouse strains, H/R stress induced a downregulation of Runx2 and Sparc (osteonectin), suggesting reduced osteoblast differentiation (Runx2) and activity (Sparc, osteonectin) (supplemental Figure 3). This was associated with upregulation of RankL and Rank, indicating increased osteoclast activity and recruitment (supplemental Figure 3).
Zol, administrated before or after H/R stress, prevented the H/R-induced increased bone turnover in both mouse strains. It is of interest to note that in SCD mice, pre-H/R administration of Zol was more efficient in reducing bone turnover than Zol administrated after H/R stress (Figure 2D). Zol treatment was also able to significantly reduce osteoclast number and activity in both mouse strains (Figure 2E).
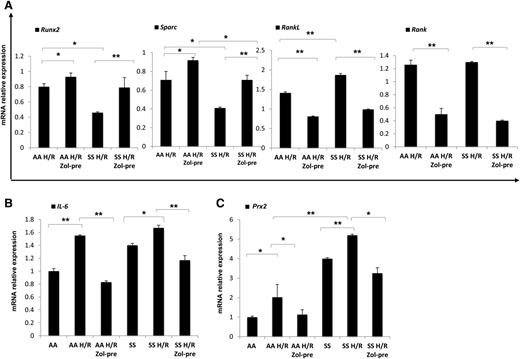
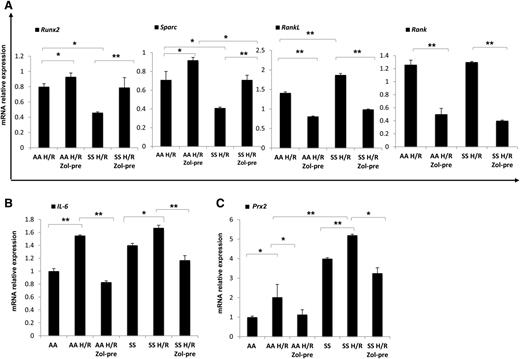
Because the administration of Zol before H/R event seemed to be more effective on bone homeostasis than after H/R, we analyzed molecular markers of osteoblast/osteoclastogenesis in mice without or with Zol treatment administrated before H/R event. Zol prevented the H/R-induced downregulation of Runx2 and Sparc and H/R-induced upregulation of RankL and Rank gene expression in both mouse strains (Figure 3A). We then analyzed IL-6 gene expression in mice exposed to H/R stress with and without Zol treatment. As shown in Figure 3B, Zol prevented the H/R-induced upregulation of IL-6 in both mouse strains, possibly through the modulation of H/R-induced increased osteoclast activity as previously reported for other aminobisphosphonates.42 With reduction in osteoclast activity and recruitment, we consistently observed a downregulation of H/R-induced increases in Prx2 expression in both mouse strains (Figure 3C). No further changes in bone microarchitecture related to a single H/R event were observed in both healthy and SCD mice (data not shown).
Hypoxia/reoxygenation stress (H/R) downregulates osteoblastogenesis, induces osteoclastogenesis, upregulates peroxiredoxin-2, anti-oxidant system. Zoledronic acid plays a preventing role in H/R-induced molecular bone expression. Runx2, Sparc, RankL, and Rank (A), IL-6 (B), and Prx2 (C) mRNA fold change in healthy (AA) and sickle cell (SS) mice exposed to H/R stress without and with Zol treatment before (pre-Zol). The fold change for each gene expression was obtained by using 2 different housekeeping genes (Gapdh and β-Actin). Data are shown as mean ± SD (n = 6); *P < .05; **P < .005.
Hypoxia/reoxygenation stress (H/R) downregulates osteoblastogenesis, induces osteoclastogenesis, upregulates peroxiredoxin-2, anti-oxidant system. Zoledronic acid plays a preventing role in H/R-induced molecular bone expression. Runx2, Sparc, RankL, and Rank (A), IL-6 (B), and Prx2 (C) mRNA fold change in healthy (AA) and sickle cell (SS) mice exposed to H/R stress without and with Zol treatment before (pre-Zol). The fold change for each gene expression was obtained by using 2 different housekeeping genes (Gapdh and β-Actin). Data are shown as mean ± SD (n = 6); *P < .05; **P < .005.
The present data suggest that each acute VOC promotes osteoclast recruitment and activation, increases bone turnover, inhibits osteogenesis, and promotes bone loss in SCD mice. Zol efficiently prevents H/R-induced bone impairment and may be considered an interesting new treatment to prevent or limit SBD.
In SCD mice, recurrent H/R events cause severe bone impairment and bone loss
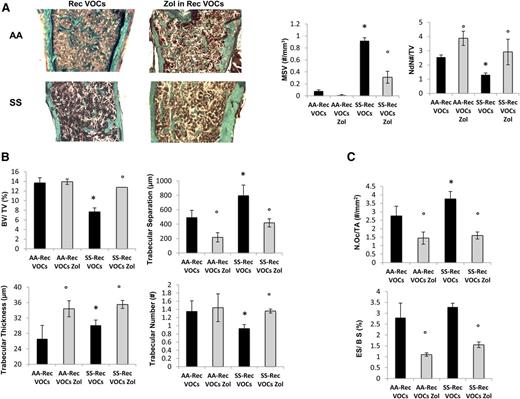
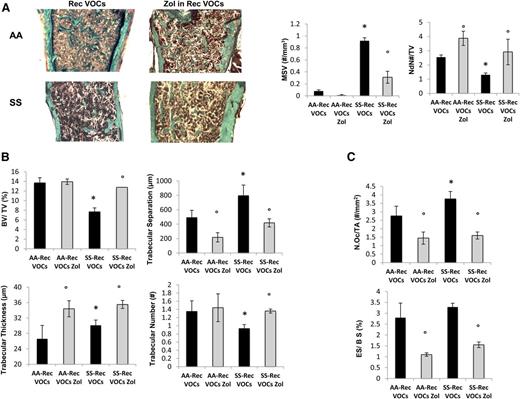
Because SCD patients experience recurrent VOCs,11 we exposed SCD and healthy mice to recurrent H/R stress (10 hours on 8% oxygen followed by reoxygenation) once every 3 weeks repeated 3 times (3 times in 9 weeks). In SCD mice, the recurrent VOCs worsened anemia and were associated with a marked increase in neutrophil count compared with either normoxic SCD mice or healthy mice exposed to recurrent H/R stress (supplemental Table 3). As shown in Figure 4A-B, recurrent VOCs severely worsened bone microarchitecture as supported by increased MSV, reduced node numbers, and node/termini ratio compared with controls (Figure 4A, right panel, and supplemental Figure 4A). SCD mice undergoing recurrent VOCs also showed reduced trabecular bone volume and number associated with increased trabecular separation compared with normal controls (Figure 4B). We did not observed major differences in osteoblast numbers and osteoid surface between the 2 mouse strains, suggesting that recurrent H/R events also affect bone homeostasis in control mice (data not shown). Osteoclast number and erosion were significantly higher in SCD mice compared with controls (Figure 4C). Interestingly, in healthy and SCD mice exposed to recurrent H/R events, the osteoclast number and erosion number were significantly higher than those observed under normoxic conditions in both mouse strains (P < .05 normoxia vs H/R, Figures 1C and 4C).
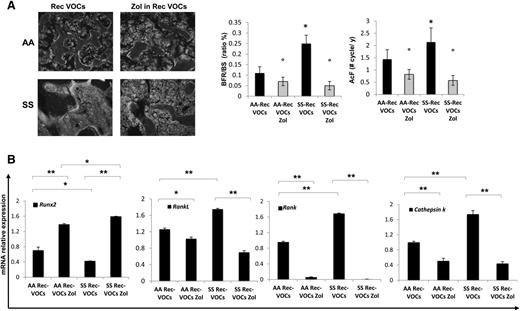
Recurrent hypoxia/reoxygenation (H/R) stress mimicking acute vaso-occlusive crisis (VOCs) worsens bone structure in sickle cell mice. (A) Quantitative histomorphometry of the distal femur of healthy (AA) and sickle cell (SS) mice exposed to recurrent (Rec) acute VOCs without and with Zol treatment. Left panel: representative undecalcified section of distal femur stained with trichrome Goldner’s stain. Right panel: MSV, marrow star volume; NdN/TV, node number/tissue volume; NdN/Tm, node to termini ratio. Data are shown as mean ± SD (n = 6); P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice. (B) Bone volume (BV)/tissue volume (TV), trabecular thickness, trabecular number, and trabecular separation in healthy (AA) and sickle cell (SS) mice exposed to recurrent (Rec) acute VOCs without and with Zol treatment. (C) N.Oc/TA, osteoclast tumber/tissue area; ES/BS, erosion surface/bone surface in AA and SS mice exposed to recurrent (Rec) acute VOCs without and with Zol. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice.
Recurrent hypoxia/reoxygenation (H/R) stress mimicking acute vaso-occlusive crisis (VOCs) worsens bone structure in sickle cell mice. (A) Quantitative histomorphometry of the distal femur of healthy (AA) and sickle cell (SS) mice exposed to recurrent (Rec) acute VOCs without and with Zol treatment. Left panel: representative undecalcified section of distal femur stained with trichrome Goldner’s stain. Right panel: MSV, marrow star volume; NdN/TV, node number/tissue volume; NdN/Tm, node to termini ratio. Data are shown as mean ± SD (n = 6); P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice. (B) Bone volume (BV)/tissue volume (TV), trabecular thickness, trabecular number, and trabecular separation in healthy (AA) and sickle cell (SS) mice exposed to recurrent (Rec) acute VOCs without and with Zol treatment. (C) N.Oc/TA, osteoclast tumber/tissue area; ES/BS, erosion surface/bone surface in AA and SS mice exposed to recurrent (Rec) acute VOCs without and with Zol. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice.
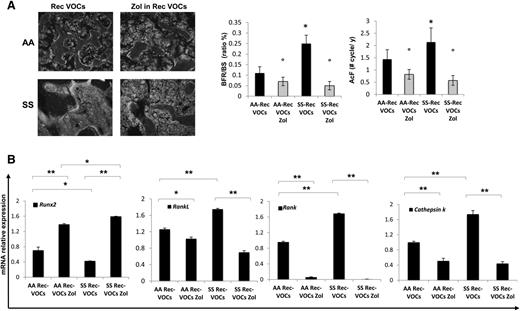
In recurrent H/R-exposed mice, calcein double-labeling revealed a higher bone turnover in SCD compared with healthy mice (Figure 5A, BFR/BS and AcF). In addition, this was higher in both mouse strains when compared with bone turnover under normoxic conditions (P < .05 normoxia vs H/R, Figures 1C and 5A).
Recurrent acute vaso-occlusive crisis (VOCs) increases bone turnover, downregulates osteoblastogenesis, and upregulates osteoclastogenesis in sickle cell mice. Zoledronic acid (Zol) prevents bone impairment induced by repeated VOCs. (A) Left panel: representative images of calcein-labeled bone surface. Right panel: bone formation rate (BFR/BS) and activation frequency (AcF) in healthy (AA) and sickle cell (SS) mice exposed to recurrent (Rec) acute VOCs without and with Zol. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice. (B) Runx2, RankL, Rank, and Cathepsin k mRNA fold changes in AA and SS mice exposed to recurrent (Rec) acute VOCs without and with Zol. Two different housekeeping genes (Gapdh and β-Actin) were used to perform real-time PCR analysis. Data are shown as mean ± SD (n = 6); *P < .05; **P < .005.
Recurrent acute vaso-occlusive crisis (VOCs) increases bone turnover, downregulates osteoblastogenesis, and upregulates osteoclastogenesis in sickle cell mice. Zoledronic acid (Zol) prevents bone impairment induced by repeated VOCs. (A) Left panel: representative images of calcein-labeled bone surface. Right panel: bone formation rate (BFR/BS) and activation frequency (AcF) in healthy (AA) and sickle cell (SS) mice exposed to recurrent (Rec) acute VOCs without and with Zol. Data are shown as mean ± SD (n = 6); *P < .05 compared with healthy mice; °P < .05 compared with vehicle-treated mice. (B) Runx2, RankL, Rank, and Cathepsin k mRNA fold changes in AA and SS mice exposed to recurrent (Rec) acute VOCs without and with Zol. Two different housekeeping genes (Gapdh and β-Actin) were used to perform real-time PCR analysis. Data are shown as mean ± SD (n = 6); *P < .05; **P < .005.
These data indicate that in SCD mice, the recurrent H/R events result in bone loss with severe alteration of bone structure and increased bone turnover. Consistently, Runx2 was downregulated, whereas RankL, Rank, and Cathepsin k were upregulated in SCD mice compared with healthy mice (Figure 5B). IL-6 and Prx2 expression were again higher in SCD mice than in controls, supporting the contribution of oxidative stress in SBD (supplemental Figure 4B).
Zoledronic acid restrains osteoclast activity, ameliorates bone microarchitecture, and prevents bone loss in SCD mice exposed to recurrent H/R events
To evaluate the impact of Zol on SBD that resulted from recurrent H/R events, we treated SCD and control mice with Zol (100 μg/kg IP, single dose) 1 month before the first of the 3 repeated VOC events. As shown in Figure 4A, Zol significantly prevented the H/R-induced microarchitecture alterations and maintained bone volume and trabecular thickness, indicating that Zol prevents bone loss and ameliorates bone microarchitecture in SCD mice (Figure 4A-B). These findings agreed with the reduction in osteoclast number and erosion surface in Zol-treated SCD mice compared with vehicle-treated mice (Figure 4C). No major differences were observed in osteoblast number in both Zol- and vehicle-treated mice (data not shown). The recurrent H/R stress–induced bone turnover was significantly decreased in both Zol-treated SCD and AA mice compared with vehicle-treated mice (Figure 5A). In addition, Zol treatment significantly increased osteoblastogenesis (Runx2) and decreased osteoclast activity (RankL, Cathepsin k) and recruitment (Rank) in both mouse strains exposed to recurrent H/R stress (Figure 5B). This was associated with downregulation of IL-6 and Prx2 (supplemental Figure 4B) expression in Zol-treated SCD mice compared with vehicle-treated mice, in agreement with the reduction of osteoclast activity.
Our results indicate that Zol prevents the development of SBD in SCD mice exposed to recurrent VOCs.
Discussion
SBD greatly affects SCD patient quality of life, and its clinical management is still unsatisfactory. Limited studies on SBD are available and its pathogenesis has been only partially investigated.18,43-47
Here we show bone impairment and bone loss in SCD mice under normoxic conditions. This was associated with downregulation of osteogenic markers (Runx2 and Sparc) and increased osteoclast activity (RankL) without major changes in osteoclastogenesis. Because Runx2 and Sparc explore osteoblast recruitment and differentiation, our findings suggest a decreased MSC commitment in osteogenic lineage in SCD mice. Indeed, we observed a significant reduction in the number of CFU-Ob from SCD mice compared with healthy controls (Figure 1E), supporting the perturbation of osteoprogenitor compartment in favor of osteoclast activity in SCD mice.
Based on this evidence, we chose Zol as a powerful inhibitor of osteoclast activity and a promoter of osteogenic differentiation to treat SBD.27,28 In normoxic SCD mice, Zol reduced osteoclast activity and improved osteoblastogenesis as supported by the downregulation of Rank and the upregulation of Runx2 and Sparc, respectively (supplemental Figure 2).
Acute vaso-occlusive events play a key role in the pathogenesis of sickle cell–related organ damage and may contribute to SBD.12 In this study, we demonstrated that H/R stress, mimicking acute VOCs, increases bone turnover, further promotes osteoclast activity (RankL) and osteoclastogenesis (Rank), and further suppresses osteogenic lineage (Runx2, Sparc) (Figure 2 and supplemental Figure 3). In SCD mice, Zol markedly prevented the H/R-induced increased bone turnover (Figure 2), osteoclast activity (RankL), and osteoclast recruitment (Rank) in favor of osteoblastogenesis (Runx2, Sparc) (Figure 3). This was associated with a downregulation of H/R-induced increased gene expression of IL-6 and Prx2, in agreement with the reduction in osteoclast activity (Figure 3B-C). Similar effects of H/R stress and Zol treatment were also observed in healthy mice, but to a lower extent compared with SCD mice. Our data strongly indicate the key role of H/R stress in the development of SBD. This is also supported by the beneficial effects of Zol, which switches off osteoclast activity and osteoclastogenesis in favor of osteoblastogenesis, limiting bone impairment related to H/R stress. It is of interest to note that the positive effects of Zol on bone homeostasis seem to be more pronounced in SCD mice treated before the exposure to H/R stress than after an H/R event.
Because SCD patients experience recurrent VOCs, we exposed SCD mice to repeated H/R stresses. We found that recurrent VOCs severely affected bone structure and increased bone turnover in SCD mice compared with either the SCD normoxic group or with SCD mice exposed to a single VOC (Figures 2, 4, and 5). This was associated with upregulation of RankL, Rank, Cathepsin k, and IL-6 as proresorptive cytokine, and downregulation of Runx2, indicating increased osteoclast recruitment and activity combined with suppression of osteoblastogenesis (Figure 5). A single dose of Zol, one month before the repeated VOCs, prevented bone impairment and bone loss, promoting osteogenic lineage in SCD mice compared with the vehicle-treated SCD group (Figures 4 and 5).
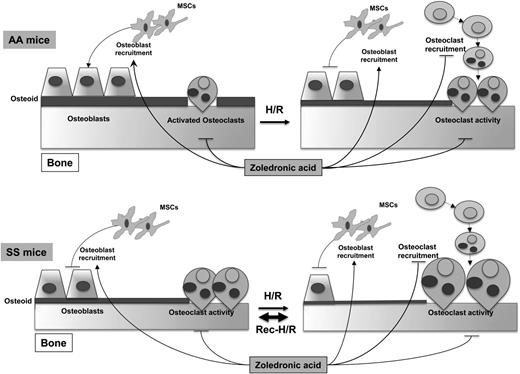
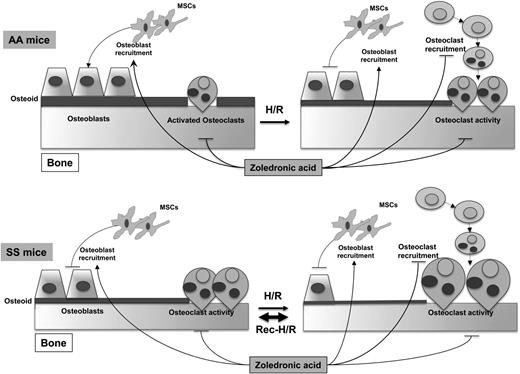
Based on this evidence, we believe that osteoblasts are the “sleeping beauty” in the pathogenesis of SBD (Figure 6). Thus, the therapeutic inhibition of osteoclast activity/osteoclastogenesis might only be partially effective in the long-term management of SBD in the absence of stimulating osteogenic lineage treatment, which might be used as a sequential therapeutic strategy after inhibitors of osteoclast activity. Data from SCD mice treated with zoledronic acid, which is a potent inhibitor of osteoclast activity with a previously reported action also on osteoblast recruitment,48 support this working model (Figure 6).
Schematic diagram of development of sickle cell bone disease (SBD) in mice. SCD mice display increased osteoclast activity and reduced osteoblastogenesis, resulting in reduced osteoid formation compared with healthy mice. H/R stress depresses osteoblastogenesis and increases osteoclast activity and osteoclastogenesis, promoting bone impairment. Recurrent H/R stresses further worsen the imbalance between osteoblastogenesis and osteoclastogenesis/osteoclast activity, resulting in bone loss and severe bone impairment. Zoledronic acid (Zol) blocks osteoclast activity and osteoclastogenesis and is associated with increased osteoblast recruitment and osteoblastogenesis, preventing the development of SBD. AA, healthy mice; H/R, hypoxia reoxygenation stress; MSCs, mesenchymal stem cells; SS, sickle cell disease.
Schematic diagram of development of sickle cell bone disease (SBD) in mice. SCD mice display increased osteoclast activity and reduced osteoblastogenesis, resulting in reduced osteoid formation compared with healthy mice. H/R stress depresses osteoblastogenesis and increases osteoclast activity and osteoclastogenesis, promoting bone impairment. Recurrent H/R stresses further worsen the imbalance between osteoblastogenesis and osteoclastogenesis/osteoclast activity, resulting in bone loss and severe bone impairment. Zoledronic acid (Zol) blocks osteoclast activity and osteoclastogenesis and is associated with increased osteoblast recruitment and osteoblastogenesis, preventing the development of SBD. AA, healthy mice; H/R, hypoxia reoxygenation stress; MSCs, mesenchymal stem cells; SS, sickle cell disease.
In conclusion, our findings support the view that SBD is related to osteoblast impairment, resulting from reduced osteoprogenitors and osteoblast activity, combined with increased osteoclast activity (Figure 6). Recurrent VOCs, associated with local oxidative stress and release of proresorptive cytokines, such as IL-6, further increase osteoclast activity and osteoclast recruitment associated with depression of osteoblastogenesis, generating a severe bone loss and bone impairment compared with either SCD normoxic or single H/R stress SCD mice (Figure 6). Zol might act on both the osteoclast and osteoblast compartments as multimodal therapy to prevent SBD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by PRIN (201228PNX83) (L.D.F.) and FUR_UNIVR (L.D.F.).
Authorship
Contribution: L.D.F. and L.D.C. designed the experiments, analyzed data, and wrote the manuscript; M.T.V. carried out molecular analysis, analyzed data, and wrote the manuscript; A. Matte' carried out the experiments and analyzed the data; L.P. carried out the analysis of iron bone content; A. Mori carried out enzyme-linked immunosorbent assays and performed bone samples preparation; A.S. carried out animal experiments; and V.S. and G.Z. carried out H/R experiments.
Conflict-of-interest disclosure: The authors declared no competing financial interests.
Correspondence: Lucia De Franceschi, Department of Medicine, University of Verona and AOUI-Verona, Policlinico GB Rossi, Piazzale L Scuro, 10, 37134 Verona, Italy; e-mail: lucia.defranceschi@univr.it; and Maria Teresa Valenti, Department of Medicine, University of Verona and AOUI-Verona, Policlinico GB Rossi, Piazzale L Scuro, 10, 37134 Verona, Italy; e-mail: mariateresa.valenti@univr.it.