Key Points
The ABC transporter, ABCC4, localizes to the platelet plasma membrane and regulates aggregation by exporting cAMP and antithrombotic drugs.
Abstract
Controlling the activation of platelets is a key strategy to mitigate cardiovascular disease. Previous studies have suggested that the ATP-binding cassette (ABC) transporter, ABCC4, functions in platelet-dense granules. Using plasma membrane biotinylation and super-resolution microscopy, we demonstrate that ABCC4 is primarily expressed on the plasma membrane of both mouse and human platelets. Platelets lacking ABCC4 have unchanged dense-granule function, number, and volume, but harbor a selective impairment in collagen-induced aggregation. Accordingly, Abcc4 knockout (KO) platelet attachment to a collagen substratum was also faulty and associated with elevated intracellular cyclic AMP (cAMP) and reduced plasma membrane localization of the major collagen receptor, GPVI. In the ferric-chloride vasculature injury model, Abcc4 KO mice exhibited markedly impaired thrombus formation. The attenuation of platelet aggregation by the phosphodiesterase inhibitor EHNA (a non-ABCC4 substrate), when combined with Abcc4 deficiency, illustrated a crucial functional interaction between phosphodiesterases and ABCC4. This was extended in vivo where EHNA dramatically prolonged the bleeding time, but only in Abcc4 KO mice. Further, we demonstrated in human platelets that ABCC4 inhibition, when coupled with phosphodiesterase inhibition, strongly impaired platelet aggregation. These findings have important clinical implications because they directly highlight an important relationship between ABCC4 transporter function and phosphodiesterases in accounting for the cAMP-directed activity of antithrombotic agents.
Introduction
Platelets play a crucial role in the pathophysiology of intravascular thrombosis, and reducing platelet aggregation is one approach to mitigate disease progression. One strategy to reduce platelet aggregation is to inhibit platelet phosphodiesterases (PDEs).1 PDEs catalyze the hydrolysis of 3′,5′-cAMP to inactive 5′-AMP by cleaving the PDE bond. This antithrombotic approach elevates the intracellular platelet cyclic AMP (cAMP) and impairs platelet aggregation. Intracellular cAMP is synthesized from adenosine triphosphate (ATP), by adenylate cyclase, to modulate platelet aggregation. Although synthesis of cAMP in platelets is stimulated by mediators as diverse as collagen and prostaglandin metabolites, the intraplatelet concentration of cAMP is tightly controlled by both synthesis and, according to convention, PDE-mediated degradation.2 The role of platelet-specific cAMP PDEs, such as PDE2 and PDE3, in regulating cAMP levels and platelet function, are well known.3 But it is not known whether ABC transporters regulate the intraplatelet concentration of cAMP or if they modulate platelet aggregation or thrombosis in response to PDE inhibitors. Specifically, we are interested in the relation between the ABC transporter ABCC4 and PDEs in platelet aggregation.
Previous reports in platelets indicated that ABCC4 localizes mostly to a platelet intracellular organelle (dense-granule), with little localized to the plasma membrane.4,5 These reports suggested that ABCC4 solely functioned as an importer of adenosine diphosphate (ADP) into platelet-dense granules, a finding based on colocalization of ABCC4 with the well-known dense-granule marker, mepacrine.4,5 However, it should be noted that, although mepacrine readily marked the dense-granules, ABCC4 localization was much less certain and ADP import, by purified dense-granules, was not demonstrated. Nonetheless, if ABCC4 was functional in dense granules, one prediction from these studies would be that loss of ABCC4 would attenuate platelet aggregation by multiple agonists, such as collagen and thrombin, both of which require ADP release from dense-granules to amplify platelet aggregation. However, it is unclear whether ABCC4 is primarily localized to dense-granules, because recent proteomic analysis of platelet plasma membranes suggests otherwise—that is, ABCC4 localizes to the plasma membrane.6 At this point, ABCC4 location in platelets is uncertain (plasma membrane, dense-granules, or both). Further, but more important, there is no direct evidence that ABCC4 functionally contributes to platelet physiology or affects response to antiplatelet medications.
In the current studies, we discovered in both human and mouse platelets that ABCC4 localizes almost exclusively to the plasma membrane. Consistent with the lack of ABCC4 in dense-granules, we show that platelets derived from Abcc4 knockout (KO) mice do not differ in the number or function of their dense-granules. Moreover, Abcc4 absence impairs collagen-induced aggregation but has no impact on thrombin or ADP-mediated aggregation. We determined that KO platelets have reduced amounts of GPVI receptor and impaired collagen-mediated signaling. Further, reduced GPVI expression is also associated with an impaired ability to bind to collagen, which is related to basal cAMP elevation and associates with impaired thrombus formation in vivo. Finally, we demonstrated for the first time in human platelets that the combination of a PDE inhibitor with an ABCC4 inhibitor produces a strong, apparently synergistic impairment in platelet aggregation. This finding is underscored by the fact that the same PDE inhibitor prolonged bleeding time, but only in treated KO mice, which is likely related to the KO platelets’ inability to efficiently export cAMP. Overall, our studies show that ABCC4 primarily localizes to the platelet plasma membrane and plays a crucial role in eliciting normal thrombus formation in vivo. These are the first studies to reveal a functional role for ABCC4 in platelet aggregation and they have strong implications for patient populations that harbor reduced or nonfunctional ABCC47,8 or those who are taking medications that are ABCC4 substrates or inhibitors.9
Methods
Structured illumination microscopy imaging
Platelet-rich plasma (PRP) from wild-type (WT) mice and a healthy human donor were seeded onto a poly-lysine–coated coverslip, fixed, and reacted with rabbit anti-Abcc4 antibody10 (1:200) and mouse anti-Na+/K+-ATPase (1:500), and images were collected with a Zeiss ELYRA PS.1 super-resolution microscope (Carl Zeiss MicroImaging).
FeCl3 carotid artery injury
One carotid artery in each isoflurane-anesthetized mouse had injury induced by 10% FeCl3 as previously described.11
The supplemental Methods, found on the Blood Web site, detail the additional methods used in this study.
Results
Analysis of ABC transporter expression in platelets
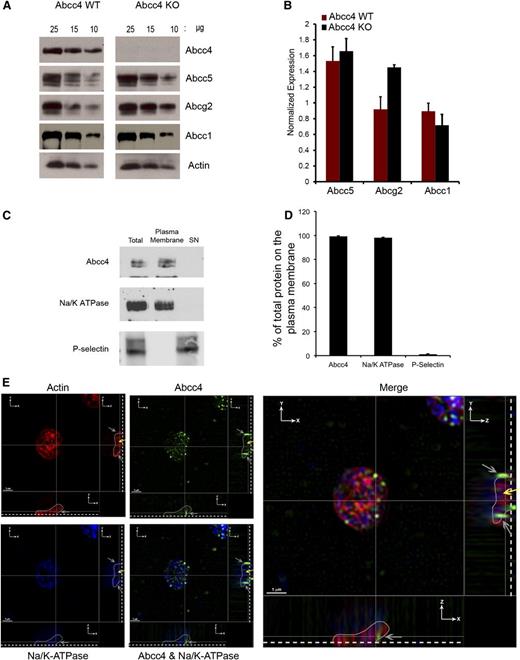
PRP was prepared from Abcc4 WT and KO mice, followed by immunoblot analysis to determine whether ABCC4 was expressed in platelets. Platelets from KO mice modestly increased Abcg2 (∼40%), but not Abcc5 (Figure 1A-B). ABCC4 localization in platelets was investigated using a combination of biochemistry and microscopy (Figure 1C-D). ABCC4 membrane localization was assessed by surface membrane biotinylation12 of freshly isolated platelets. Platelets were incubated with a membrane-impermeable succinimide ester of biotin (NHS-SS-biotin) and lysed. The biotin-labeled membranes were separated from the intracellular contents with streptavidin beads. Avidin-bead–bound platelet membranes were analyzed by immunoblot assay for ABCC4 expression (Figure 1C-D). The amount of Abcc4 at the plasma membrane was quantified and accounted for >95% of the ABCC4 in the total platelet lysate, a value that was similar to the well-known plasma membrane protein, Na+/K+-ATPase. Furthermore, the granule marker P-selectin was only detected in the internal fraction (labeled “SN”), and no ABCC4 was detectable in the internal contents. These findings indicate that ABCC4 is almost exclusively at the plasma membrane.
Mouse/ABCC4 expression at the platelet plasma membrane. (A) Immunoblots of whole-platelet lysates, loaded at the indicated concentrations, derived from Abcc4 WT and KO mice probed with Abcc4, Abcg2, Abcc5, Abcc1, and actin antibodies. (B) After densitometry and actin normalization, the amount of each protein was quantified. The error bar represents one standard deviation from n = 3 independent samples. (C) Immunoblot of biotinylated membranes bound with streptavidin beads and intracellular contents (SN) prepared from mouse Abcc4 WT platelets and quantified and plotted as a bar graph. Each experiment was performed 3 times with PRP pooled from 3 to 5 mice for each genotype. (D) Relative amount of plasma membrane ABCC4, Na+/K+-ATPase, and P-selectin was determined by densitometry from multiple individual samples (n = 4) with the error bar indicated. (E) Super resolution structured illumination microscopy (SIM) showing localization of ABCC4 (green), Na+/K+-ATPase (blue), and actin (Red). Two cross-sectional views (XZ and YZ) show ABCC4 localized with Na+/K+-ATPase at the plasma membrane (gray arrows). The dashed white line demarcates the point of platelet coverslip attachment, the white line represents a tracing that outlines the platelet shape (see supplemental Figure 1A), and the yellow arrow represents the intracellular portion of the platelet identified by actin staining. The scale bar corresponds to 1 μm for all 3 views.
Mouse/ABCC4 expression at the platelet plasma membrane. (A) Immunoblots of whole-platelet lysates, loaded at the indicated concentrations, derived from Abcc4 WT and KO mice probed with Abcc4, Abcg2, Abcc5, Abcc1, and actin antibodies. (B) After densitometry and actin normalization, the amount of each protein was quantified. The error bar represents one standard deviation from n = 3 independent samples. (C) Immunoblot of biotinylated membranes bound with streptavidin beads and intracellular contents (SN) prepared from mouse Abcc4 WT platelets and quantified and plotted as a bar graph. Each experiment was performed 3 times with PRP pooled from 3 to 5 mice for each genotype. (D) Relative amount of plasma membrane ABCC4, Na+/K+-ATPase, and P-selectin was determined by densitometry from multiple individual samples (n = 4) with the error bar indicated. (E) Super resolution structured illumination microscopy (SIM) showing localization of ABCC4 (green), Na+/K+-ATPase (blue), and actin (Red). Two cross-sectional views (XZ and YZ) show ABCC4 localized with Na+/K+-ATPase at the plasma membrane (gray arrows). The dashed white line demarcates the point of platelet coverslip attachment, the white line represents a tracing that outlines the platelet shape (see supplemental Figure 1A), and the yellow arrow represents the intracellular portion of the platelet identified by actin staining. The scale bar corresponds to 1 μm for all 3 views.
To visualize ABCC4 in mouse platelets, structured illumination microscopy (SIM) was performed on fixed and permeabilized platelets. Platelets were incubated with antibodies against ABCC4 (green), a membrane protein, Na+/K+-ATPase (blue), and actin (red). To localize Abcc4 in 3 dimensions, stepwise images of the XZ and YZ geometric planes were acquired in 110-nm Z-step increments, through the platelets (Figure 1E). The blue-labeled Na+/K+-ATPase reveals the plasma membrane of the platelet, and the outline of the platelet, as determined by 3D reconstruction using Imaris software (supplemental Figure 1A-B), is depicted in both the XZ and ZY planes with the base of the platelet indicated by the dashed line. The merged image shows ABCC4 is at the plasma membrane (gray arrows) of mouse platelets and the yellow arrow indicates the location of intracellular actin.
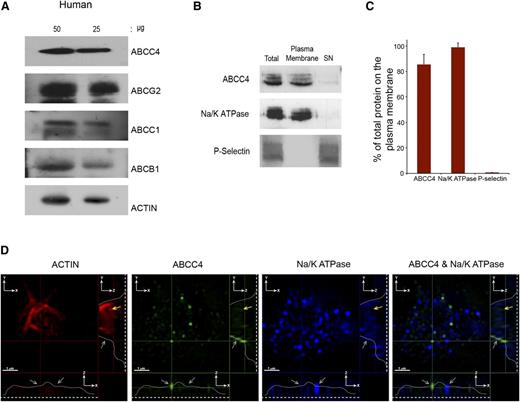
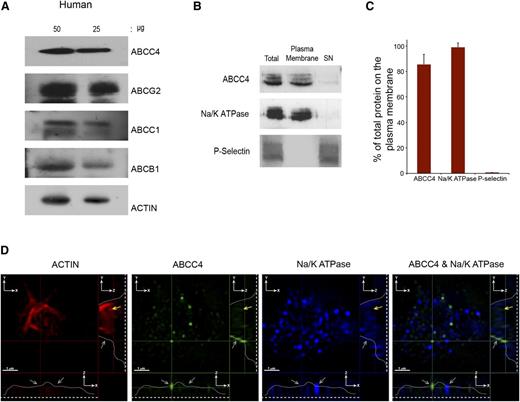
PRP prepared from a healthy donor was immunoblotted and revealed ABCC4, ABCG2, ABCC1, and ABCB1 expression in human platelets (Figure 2A). Like the murine platelets, surface biotinylation confirmed that the majority of human ABCC4 resides on the plasma membrane (∼85%) (Figure 2B-C). Notably, the plasma membrane marker, Na+/K+-ATPase, also colocalizes with ABCC4, further confirming the plasma membrane localization of ABCC4, while being undetectable in the internal cellular (SN) fraction. Moreover, the granule marker, P-selectin, was not detected in the membrane fraction. Three-dimensional SIM was performed on fixed and permeabilized human platelets. The actin staining (red) highlights the intracellular architecture of the platelet and is identified by the yellow arrow. The blue color indicates the plasma membrane marker Na+/K+-ATPase. The green highlights human ABCC4, and the merged image shows that, like mouse Abcc4, human ABCC4 localized to the plasma membrane (Figure 2D and supplemental Figure 1C-D; right and lower panel, the gray line identifies ABCC4 location in 2 planes, the gray arrows identify membrane localized Abcc4/Na+/K+-ATPase, and the yellow arrows identify internalized actin staining).
Human ABCC4 is expressed at the plasma membrane. (A) Immunoblots of whole-platelet lysates loaded at the indicated concentrations, derived from healthy human donor and probed with ABCC4, ABCG2, ABCB1, ABCC1, and actin antibodies. (B) A representative immunoblot of biotinylated plasma membrane bound to streptavidin beads and the intracellular contents (SN) prepared from human platelets probed with human ABCC4, Na/K ATPase, and P-selectin antibodies. (C) After densitometry and normalization by total the amount in each fraction was expressed as a proportion of total. (D) The proportion of ABCC4, Na+/K+-ATPase, and P-selectin was quantified by densitometry and plotted as a bar graph. Each experiment was performed at least 3 times with the error bar representing one standard error of the mean. (D) Super resolution using structured illumination microscopy (SIM) to localize proteins stained with antibodies to human ABCC4 (green), Na+/K+-ATPase (blue), and actin (red). Two cross-sectional views (XZ and YZ) show plasma membrane localized ABCC4 (gray arrows). The dashed white line represents the junction where the platelet attaches to the coverslip, whereas the white line traces the outline of the platelet shape, and the yellow arrow represents the intracellular portion of the platelet defined by actin staining and devoid of ABCC4 staining. The scale bar corresponds to 1 μm for all 3 views.
Human ABCC4 is expressed at the plasma membrane. (A) Immunoblots of whole-platelet lysates loaded at the indicated concentrations, derived from healthy human donor and probed with ABCC4, ABCG2, ABCB1, ABCC1, and actin antibodies. (B) A representative immunoblot of biotinylated plasma membrane bound to streptavidin beads and the intracellular contents (SN) prepared from human platelets probed with human ABCC4, Na/K ATPase, and P-selectin antibodies. (C) After densitometry and normalization by total the amount in each fraction was expressed as a proportion of total. (D) The proportion of ABCC4, Na+/K+-ATPase, and P-selectin was quantified by densitometry and plotted as a bar graph. Each experiment was performed at least 3 times with the error bar representing one standard error of the mean. (D) Super resolution using structured illumination microscopy (SIM) to localize proteins stained with antibodies to human ABCC4 (green), Na+/K+-ATPase (blue), and actin (red). Two cross-sectional views (XZ and YZ) show plasma membrane localized ABCC4 (gray arrows). The dashed white line represents the junction where the platelet attaches to the coverslip, whereas the white line traces the outline of the platelet shape, and the yellow arrow represents the intracellular portion of the platelet defined by actin staining and devoid of ABCC4 staining. The scale bar corresponds to 1 μm for all 3 views.
Morphology of WT and Abcc4 KO platelets
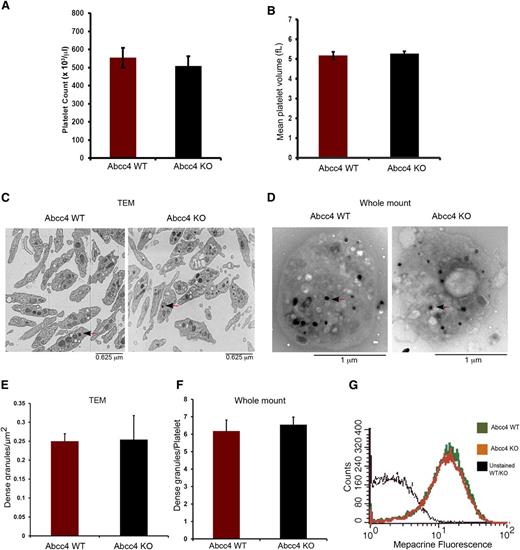
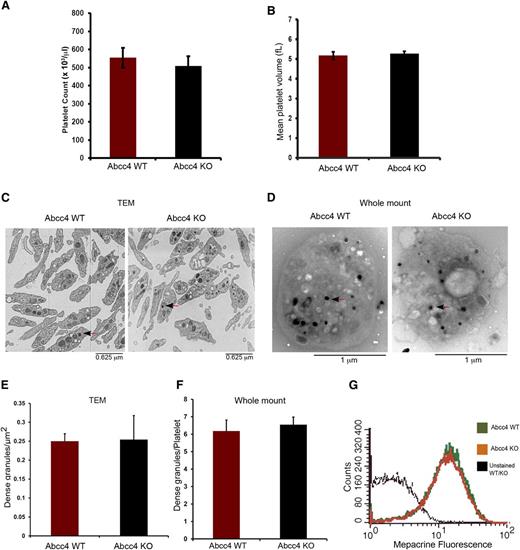
Complete blood counts (CBC) revealed that WT and KO mice had no significant differences in platelet number (554.75 ± 54.82, 508.25 ± 74.55 platelets/μL for WT and KO, respectively) (Figure 3A). Similarly, the mean platelet volume of WT (5.167 ± 0.189 fL) and KO (5.267 ± 0.125 fL) platelets was almost identical (Figure 3B). Transmission electron microscopy of fixed WT and KO platelets revealed no morphologic differences and similar numbers of dense-granules/μm2 (Figure 3C,E). We corroborated these findings by performing electron microscopy using unfixed, unstained whole-mount platelets (Figure 3D,F). This approach also showed that, between the KO and WT platelets, the number of platelet-dense granules were almost identical.
Abcc4 WT and KO platelets have similar morphology. There is no difference in Abcc4 WT and KO platelet count (A) and volume (B) as determined from the complete blood count of blood. (C) Transmission electron micrographs of fixed Abcc4 WT and KO platelets. The arrow identifies the dense granules. (D) Whole-mount electron micrographs of Abcc4 WT and KO platelets with arrows (black arrowhead and red stem) indicating the dense-granules. (E) Quantification of the number of dense-granules (represented as per μm2) from the fixed platelets using transmission electron microscopy. (F) The number of dense-granules per platelet from unfixed whole-mount EMs of Abcc4 WT and KO represented as dense-granule per platelet. (G) A representative experiment of mepacrine accumulation measured by flow cytometry in Abcc4 WT and KO platelets (n = 3). Platelet count and volume data were derived from 10 to 12 mice per group and repeated twice. Transmission electron microscopy was performed with pooled platelets from 3 mice in each group. The quantification is acquired from 10 platelets. The bars in each figure represent 1 standard deviation.
Abcc4 WT and KO platelets have similar morphology. There is no difference in Abcc4 WT and KO platelet count (A) and volume (B) as determined from the complete blood count of blood. (C) Transmission electron micrographs of fixed Abcc4 WT and KO platelets. The arrow identifies the dense granules. (D) Whole-mount electron micrographs of Abcc4 WT and KO platelets with arrows (black arrowhead and red stem) indicating the dense-granules. (E) Quantification of the number of dense-granules (represented as per μm2) from the fixed platelets using transmission electron microscopy. (F) The number of dense-granules per platelet from unfixed whole-mount EMs of Abcc4 WT and KO represented as dense-granule per platelet. (G) A representative experiment of mepacrine accumulation measured by flow cytometry in Abcc4 WT and KO platelets (n = 3). Platelet count and volume data were derived from 10 to 12 mice per group and repeated twice. Transmission electron microscopy was performed with pooled platelets from 3 mice in each group. The quantification is acquired from 10 platelets. The bars in each figure represent 1 standard deviation.
A previous study suggested that ABCC4 was expressed in dense-granules.4 Impaired mepacrine uptake13 is characteristic of defective dense-granules. Before assessing mepacrine uptake, platelet size was determined because mepacrine uptake relates to platelet size.13 The KO and WT platelets had similar sizes and volumes. KO and WT dense-granules accumulated almost identical amounts of mepacrine, thus demonstrating that ABCC4 absence does not impair dense-granule function (Figure 3G).
Analysis of aggregation of Abcc4 WT and KO platelets
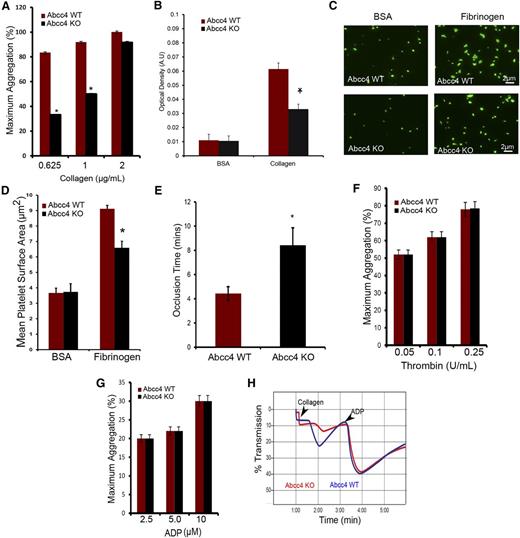
Aggregation was assessed in PRP derived from both WT and KO mice to investigate platelet function. The first agonist tested was collagen (Figure 4A and supplemental Figure 2A-C), which relies on the heterodimeric receptor complex, GPVI and FcRγ.14 At the lowest collagen concentration (0.625 μg/mL), KO platelets achieved aggregation of only ∼33% of WT. However, at the highest collagen concentration (2 μg/mL), KO platelets responded similarly to WT platelets (Figure 4A). Platelets were tested for adhesion to slides coated with monomeric collagen. Under these static conditions, KO platelet attachment was markedly impaired (Figure 4B). To investigate spreading, WT and KO platelets were first attached to either fibrinogen-coated slides or, as a control, bovine serum antigen–coated slides. KO platelet spreading is significantly reduced on fibrinogen (KO platelets [6.58 μm2] vs WT platelets [9.11 μm2]) with no spreading differences on bovine serum antigen (Figure 4C-D).
Abcc4 KO platelets have reduced aggregation with reduced thrombus formation in vivo. (A) Collagen-mediated, Abcc4 WT, and KO platelet aggregation measured using PRP in a Chronolog aggregometer with various concentrations of collagen. Platelet aggregation was performed 3 times with PRP pooled from 3 to 5 mice for each genotype. The bar represents 1 standard deviation. (B) PRP was used to determine the platelet binding to collagen-coated plates and the optical density (AU) (*P < .05). The binding assay was performed twice and the data were derived from pooled PRP of 3 to 5 individual mice per genotype per experiment. The bar represents 1 standard deviation. (C) Representative image of the spreading of Abcc4 KO and WT platelets on fibrinogen-coated coverslips. (D) The surface area covered by each platelet was determined for 200 platelets and 10 independent fields. This experiment was repeated twice with pooled PRP, with the data derived from 3 individual mice of each genotype shown. The bar represents 1 standard deviation for platelet surface area. (E) The mean time to vessel occlusion was calculated from FeCl3 experiments performed on 5 to 8 mice per group. (F) Percentage of thrombin-mediated platelet aggregation measured using Abcc4 WT and KO-derived PRP by a Chronolog aggregometer after incubation with various thrombin concentrations. (G) Percentage of ADP-mediated platelet aggregation measured using derived PRP. (H) A representative aggregation tracing of PRP from Abcc4 WT and KO mouse treated first with 0.625 μg/mL collagen and, at the indicated time, 1.25 μΜ ADP. Platelet aggregation experiments were performed 3 times using PRP derived from 3 to 5 individual mice per genotype. The bar in each graph represents 1 standard deviation (*P value < .05).
Abcc4 KO platelets have reduced aggregation with reduced thrombus formation in vivo. (A) Collagen-mediated, Abcc4 WT, and KO platelet aggregation measured using PRP in a Chronolog aggregometer with various concentrations of collagen. Platelet aggregation was performed 3 times with PRP pooled from 3 to 5 mice for each genotype. The bar represents 1 standard deviation. (B) PRP was used to determine the platelet binding to collagen-coated plates and the optical density (AU) (*P < .05). The binding assay was performed twice and the data were derived from pooled PRP of 3 to 5 individual mice per genotype per experiment. The bar represents 1 standard deviation. (C) Representative image of the spreading of Abcc4 KO and WT platelets on fibrinogen-coated coverslips. (D) The surface area covered by each platelet was determined for 200 platelets and 10 independent fields. This experiment was repeated twice with pooled PRP, with the data derived from 3 individual mice of each genotype shown. The bar represents 1 standard deviation for platelet surface area. (E) The mean time to vessel occlusion was calculated from FeCl3 experiments performed on 5 to 8 mice per group. (F) Percentage of thrombin-mediated platelet aggregation measured using Abcc4 WT and KO-derived PRP by a Chronolog aggregometer after incubation with various thrombin concentrations. (G) Percentage of ADP-mediated platelet aggregation measured using derived PRP. (H) A representative aggregation tracing of PRP from Abcc4 WT and KO mouse treated first with 0.625 μg/mL collagen and, at the indicated time, 1.25 μΜ ADP. Platelet aggregation experiments were performed 3 times using PRP derived from 3 to 5 individual mice per genotype. The bar in each graph represents 1 standard deviation (*P value < .05).
The FeCl3-induced thrombus formation assay11 investigated whether thrombus formation is altered in KO mice, with blood flow measuring the time to arterial occlusion (Figure 4E). KO mice exhibited a significantly delayed average occlusion time (8.45 minutes) with FeCl3 compared with the WT (4.43 minutes, P < .05) (representative traces are displayed in supplemental Figure 3).
Because thrombin has been implicated in some of the response to FeCl3, we tested KO platelet response to thrombin, because it is reportedly modulated by cAMP15 (Figure 4F). Thrombin-elicited aggregation was almost identical between WT and KO platelets (Figure 4F and supplemental Figure 2D-F). KO platelets exhibited no defect in aggregation upon challenge with the P2Y12 receptor agonist, ADP (Figure 4G and supplemental Figure 2G-I), and P2Y12 expression was unchanged (supplemental Figure 2J-K). To confirm that the collagen aggregation defect was inherent to KO platelets but distinct from the ADP response, WT or KO platelets were treated sequentially, first with collagen (1 μg/mL) followed by ADP (1.25 μM). As expected, KO platelets exhibited defective collagen aggregation. However, addition of ADP to these same platelets revealed a similar aggregation profile in both KO and WT platelets (Figure 4H). In total, these results indicate that KO platelets do not harbor a general aggregation defect. These findings show that the impaired thrombotic response in vivo closely aligns with the attenuated in vitro response to collagen in KO platelets.
Collagen receptor GPVI expression is reduced in Abcc4 KO platelets
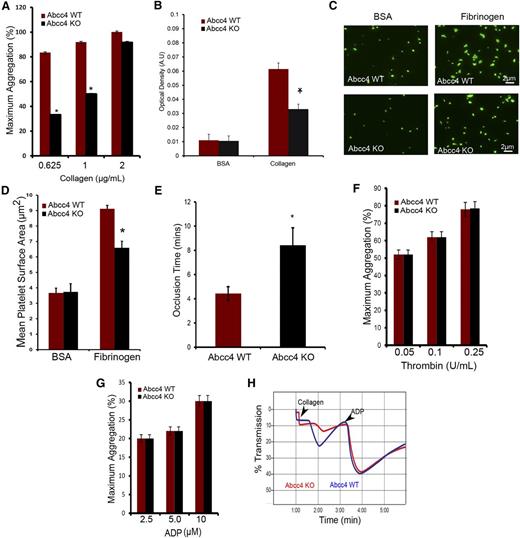
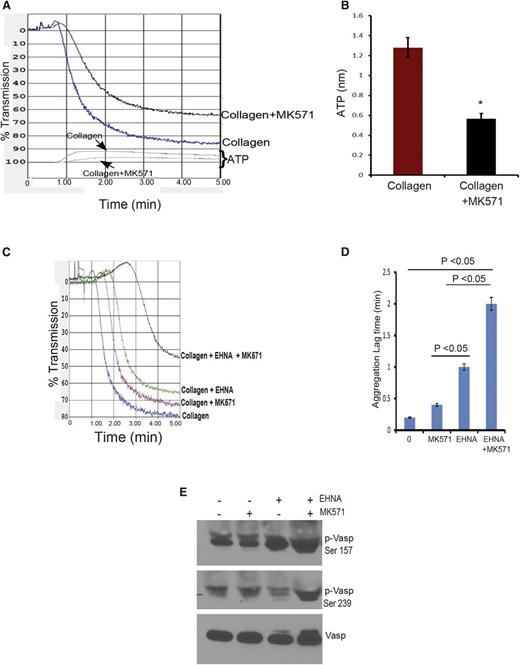
Platelet membrane protein GPVI is a primary receptor for collagen,16,17 where it heterodimerizes with FcRγ, leading to Syk recruitment, to produce Plcγ2 activation.18 To investigate whether reduced collagen-mediated aggregation is caused by altered expression of this pathway in KO platelets, we determined the levels of these proteins (Figure 5A-B). Immunoblot analysis of platelet lysates showed a 60% reduction in the amount of GPVI in the KO platelets (Figure 5C). Further, surface biotinylation demonstrated that the proportion of GPVI at the cell surface was reduced 25% in the KO (Figure 5C-D), a value strikingly similar to 25% reduction obtained by fluorescence-activated cell sorting analysis with an antibody that detects GPVI on the surface (supplemental Figure 4A-B). Thus, the reduction in GPVI available to collagen in the KO platelets parallels the defect in collagen-mediated aggregation and attachment. Reduced GPVI is not associated with a reduction in its heterodimeric partner, FcRγ. Furthermore, amounts of other plasma membrane proteins (eg, Abcg2, Abcc5) were not decreased in KO platelets (Figure 1A). The almost identical level of the signaling molecules, Plcγ2 and Syk, in the WT and KO platelets indicated that these major downstream effectors of GPVI activation19 were not reduced (Figure 5E-F). Intracellular cAMP concentration in both WT and KO platelets revealed that basal KO platelet cAMP concentration was 60% greater at 8 pmol per 2.0 × 107 platelets vs 5 pmol per 2.0 × 107 in WT platelets (Figure 5G). Furthermore, activating adenylate cyclase with PGE1 revealed that internal cAMP was more than twofold more abundant in KO platelets (Figure 5H), a finding consistent with defective cAMP export.
Abcc4 KO platelets have decreased GPVI expression. (A) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets, loaded at the indicated concentrations, and probed with either GPVI or its binding partner FcRγ. (Β) After densitometry and normalization for loading, the signals were quantified (*P < .05). (C) Immunoblot analysis of total-platelet lysate and surface membrane proteins determined by biotinylation of mouse Abcc4 WT and KO platelets. The transferrin receptor (TFR) was used as a membrane control. (D) After densitometry and normalization by total lysate (Total), the percent on the membrane surface was determined. The error bar represents the standard error of the mean of 3 experiments. (E) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets loaded at the indicated concentrations and probed with Plcγ2, Syk, and actin antibodies. (F) After normalization by actin, the relative amount of Plcγ2 and Syk is shown. (G) Basal cAMP levels in Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05. (H) Ratio of internal vs external cAMP levels post–PGE1 stimulation in the Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05). (I) Immunoblot of platelet-surface membrane proteins obtained after surface biotinylation and avidin-bead pull-down from Abcc4 WT platelets, either untreated or treated with 10 μM of forskolin. Immunoblots were probed with GPVI, as the loading control blot was stained with ponceau S (I, input from total lysate; B, bound to the avidin-bead [ie, surface protein and S in supernatant or cytosol]). (J) Representative Vasp and phospho-Vasp immunoblots in platelet lysates prepared from Abcc4 WT and KO treated with either 1 or 2 μg of collagen, 0.01 U of thrombin, or 10 μM of forskolin. Blots were probed with p-Vasp Ser 157, p-Vasp Ser 239, and an antibody that recognizes total Vasp. Phosphorylation was determined for Ser 157 (K) or Ser 239 (L) as percentage of forskolin (Fsk)-induced phosphorylation. Platelet lysate immunoblot analysis is performed 3 times using pooled PRP-derived platelets from 2 to 4 mice per genotype. The bar in the graph represents 1 standard deviation. Untxt, untreated.
Abcc4 KO platelets have decreased GPVI expression. (A) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets, loaded at the indicated concentrations, and probed with either GPVI or its binding partner FcRγ. (Β) After densitometry and normalization for loading, the signals were quantified (*P < .05). (C) Immunoblot analysis of total-platelet lysate and surface membrane proteins determined by biotinylation of mouse Abcc4 WT and KO platelets. The transferrin receptor (TFR) was used as a membrane control. (D) After densitometry and normalization by total lysate (Total), the percent on the membrane surface was determined. The error bar represents the standard error of the mean of 3 experiments. (E) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets loaded at the indicated concentrations and probed with Plcγ2, Syk, and actin antibodies. (F) After normalization by actin, the relative amount of Plcγ2 and Syk is shown. (G) Basal cAMP levels in Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05. (H) Ratio of internal vs external cAMP levels post–PGE1 stimulation in the Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05). (I) Immunoblot of platelet-surface membrane proteins obtained after surface biotinylation and avidin-bead pull-down from Abcc4 WT platelets, either untreated or treated with 10 μM of forskolin. Immunoblots were probed with GPVI, as the loading control blot was stained with ponceau S (I, input from total lysate; B, bound to the avidin-bead [ie, surface protein and S in supernatant or cytosol]). (J) Representative Vasp and phospho-Vasp immunoblots in platelet lysates prepared from Abcc4 WT and KO treated with either 1 or 2 μg of collagen, 0.01 U of thrombin, or 10 μM of forskolin. Blots were probed with p-Vasp Ser 157, p-Vasp Ser 239, and an antibody that recognizes total Vasp. Phosphorylation was determined for Ser 157 (K) or Ser 239 (L) as percentage of forskolin (Fsk)-induced phosphorylation. Platelet lysate immunoblot analysis is performed 3 times using pooled PRP-derived platelets from 2 to 4 mice per genotype. The bar in the graph represents 1 standard deviation. Untxt, untreated.
Elevated cAMP lowered GPVI surface expression
Abcc4 WT platelets were incubated with forskolin to investigate whether intracellular cAMP elevation alters GPVI membrane localization. The plasma membrane surface was covalently labeled with a succinimide-ester of biotin and the proportion of GPVI on the plasma membrane quantified. The total amount of GPVI was unaffected by adenylate cyclase activation (Figure 5I), but the amount of plasma membrane GPVI was reduced more than fourfold. The intracellular GPVI increased almost 80% after forskolin treatment, a finding suggesting that elevated cAMP either increases GPVI endocytosis or blocks its membrane localization.
Collagen-mediated Vasp phosphorylation is reduced in Abcc4 KO platelets
Phosphorylation of Vasp at Ser 157 and Ser 239 can be a surrogate for protein kinase A (PKA) activation by cAMP.20,21 We hypothesized that KO platelets might exhibit altered Vasp phosphorylation in response to collagen. To evaluate this, WT and KO platelets were incubated with collagen. The PKA pathway was interrogated by treating platelets with forskolin. Forskolin-treated platelets from both KO and WT mice exhibited strong Vasp phosphorylation at both Ser 157 and Ser 239, indicating that the PKA pathway was not generally defective in the KO platelets (Figure 5J-L). In contrast, collagen-activated KO platelets displayed only 4% of total Vasp phosphorylation at Ser 239, whereas WT had an almost tenfold higher Ser 239 phosphorylation at 39%. Vasp phosphorylation was also much higher at Ser 157 for WT platelets (98%) compared with KO platelets (39%) (Figure 5J-L). Incidentally, although KO platelets do not have a defect in thrombin-induced aggregation (Figure 4F), Ser 157 and Ser 239 phosphorylation is reduced. In total, ABCC4 absence produced defects downstream of GPVI that are related to PKA signaling; however, the absence of ABCC4 was not associated with defective PKA activation.
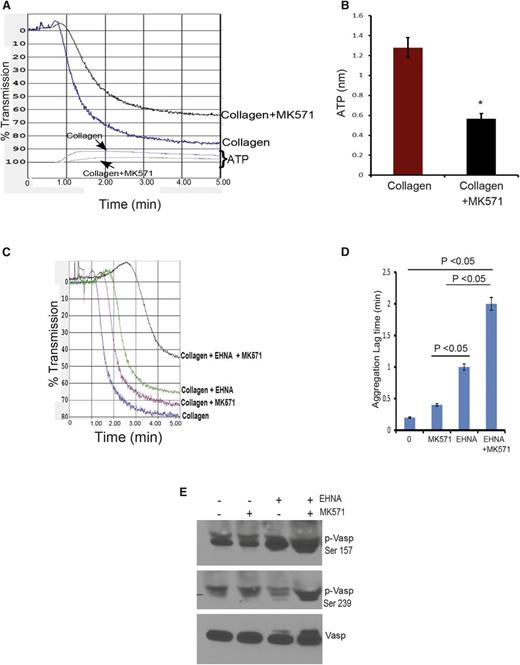
Interaction between PDE inhibitor and ABCC4
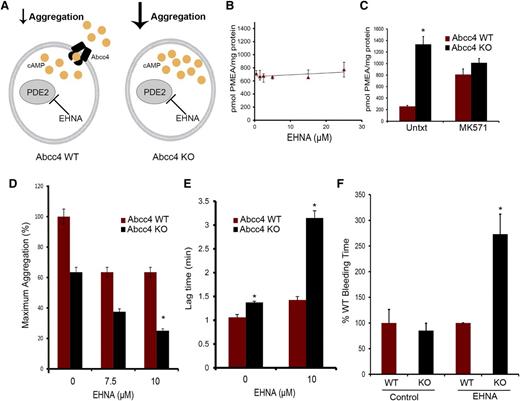
We screened PDE inhibitors to identify those that did not interact with ABCC4 to further investigate ABCC4 contribution to cAMP modulation. The relationship depicted in Figure 6A illustrates how PDE2 inhibition, along with ABCC4, might modulate cAMP. For example, one proposition is that if Abcc4 has no role in collagen-induced platelet aggregation, then both KO and WT will exhibit identical aggregation responses when PDE2 is inhibited. To identify compounds interacting with ABCC4, we used mouse embryonic fibroblast (MEF) cell lines derived from WT and KO mice.9,22 Our criteria were that increased intracellular retention of the ABCC4 substrate, 9-(2-phosphonomethoxyethyl)adenine (PMEA), defined drugs that interacted with ABCC4, whereas those that did not were neither Abcc4 substrates nor inhibitors.23 As shown in Figure 6B, the PDE2 inhibitor, erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA) did not increase PMEA accumulation. As a positive control, the ABCC4 inhibitor, MK571, increased PMEA accumulation (Figure 6C), but, as expected, only in the WT MEFs. Subsequently, KO and WT platelets were treated with EHNA, followed by collagen. As expected, in the absence of EHNA, collagen-stimulated aggregation of KO platelets was only 60% of the maximum attained by WT platelets (Figure 6D). WT platelets did not display enhanced collagen-mediated aggregation as the dose of EHNA increased (Figure 6D). In contrast, KO platelets exhibited increasingly impaired aggregation as EHNA concentrations increased. The attenuated aggregation in the KO platelets was underscored by their marked 2.5-fold lag in initiating aggregation when EHNA was present (from 1.4 to 3.2 minutes) (Figure 6E). The combined role of ABCC4 and PDE was supported by findings in WT platelets whereby increased concentrations of EHNA had no impact on collagen-induced aggregation. This is consistent with the idea that ABCC4 exports the “overflow” of intracellular cAMP produced when PDE2 is inhibited by EHNA, something the KO platelets are incapable of performing.
ABCC4 interacts with phosphodiesterase to affect aggregation and bleeding time. (A) Model depicting how ABCC4 affects platelet cAMP (yellow circle) and aggregation after treatment with the PDE2 inhibitor, EHNA. (B) ABCC4 function was assessed with a PMEA accumulation assay conducted in the presence and absence of either EHNA or (C) the bona fide ABCC4 inhibitor, MK571, using Abcc4 WT and KO-derived MEF cell lines (P < .05) (n = 5). (D) Maximum platelet aggregation of Abcc4 WT and KO platelets after blocking PDE2 activity with various concentrations of EHNA. (E) Lag time in Abcc4 WT and KO platelet aggregation after blocking PDE2 activity with EHNA. (F) Mice were treated with either vehicle or EHNA, and tail bleeding time was determined 30 minutes after drug administration. Abcc4 WT (n = 7) and Abcc4 KO (n = 5) (*P value < .01). The platelet aggregation data were derived from experiments performed at least 3 times using pooled PRP derived from 3 to 5 mice for each genotype. P < .05. Untxt, untreated.
ABCC4 interacts with phosphodiesterase to affect aggregation and bleeding time. (A) Model depicting how ABCC4 affects platelet cAMP (yellow circle) and aggregation after treatment with the PDE2 inhibitor, EHNA. (B) ABCC4 function was assessed with a PMEA accumulation assay conducted in the presence and absence of either EHNA or (C) the bona fide ABCC4 inhibitor, MK571, using Abcc4 WT and KO-derived MEF cell lines (P < .05) (n = 5). (D) Maximum platelet aggregation of Abcc4 WT and KO platelets after blocking PDE2 activity with various concentrations of EHNA. (E) Lag time in Abcc4 WT and KO platelet aggregation after blocking PDE2 activity with EHNA. (F) Mice were treated with either vehicle or EHNA, and tail bleeding time was determined 30 minutes after drug administration. Abcc4 WT (n = 7) and Abcc4 KO (n = 5) (*P value < .01). The platelet aggregation data were derived from experiments performed at least 3 times using pooled PRP derived from 3 to 5 mice for each genotype. P < .05. Untxt, untreated.
To determine whether this effect occurred in vivo, we administered EHNA, followed by bleeding time assessment, to extend the in vitro findings (Figure 6F). EHNA-treated KO mice have significantly longer bleeding time at 2.6 ± 0.41 minutes vs 0.95 ± 0.3 (P = .0043) for the WT mice (Figure 6F), a finding consistent with Abcc4 that strongly affects collagen-induced aggregation in vivo.
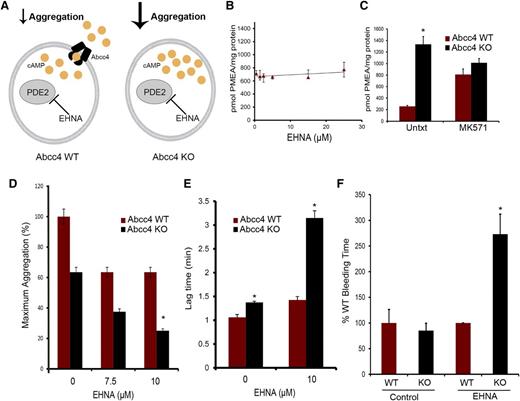
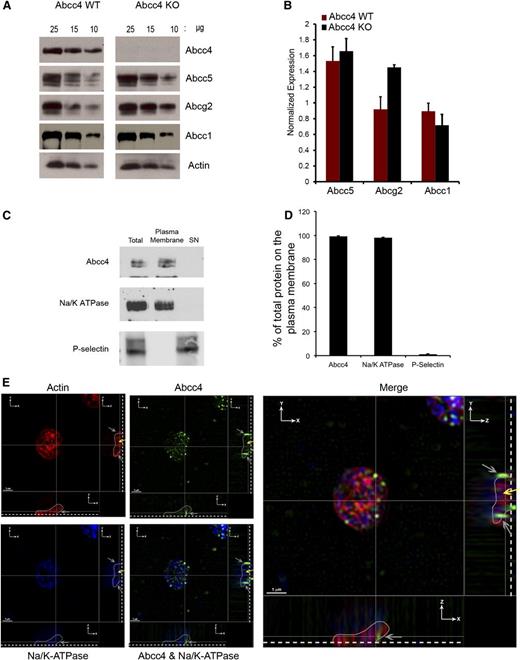
Cilostazol—a substrate for ABCC4
In contrast to EHNA, our PDE inhibitor screen revealed that the PDE3 inhibitor, cilostazol, strongly blocked PMEA export (Figure 7A). To confirm that Abcc4 export was affected, we preloaded Abcc4 WT and KO MEFs with radiolabeled PMEA, as described.24 Subsequently, cells were washed, replenished with PMEA-free medium, and both the amount of PMEA exported from and retained, were determined in the cells25 (Figure 7B). Cilostazol increased PMEA retention and blocked its export. These studies suggest that cilostazol is a competitive inhibitor/substrate of ABCC4. This is supported by the findings that cilostazol treatment did not impair collagen-mediated aggregation of WT platelets (Figure 7C). In contrast, collagen-mediated aggregation of KO platelets was markedly attenuated by cilostazol, producing only 20% of maximal aggregation (Figure 7C). A model depicting the relation between cAMP, cilostazol and ABCC4 is shown in Figure 7D. If cilostazol was an ABCC4 substrate, it might affect cAMP levels in platelets. To test whether cAMP homeostasis is differentially affected by ABCC4, we investigated Vasp phosphorylation after KO or WT platelets were incubated with cilostazol (Figure 7E). A positive control known to produce Vasp phosphorylation via PKA was forskolin. WT platelets exhibited a ninefold increase in Vasp phosphorylation, whereas KO platelets increased by almost 25-fold, a finding consistent with elevated levels of cilostazol in the KO platelets (Figure 7F).
The phosphodiesterase inhibitor cilostazol is an ABCC4 inhibitor that affects platelet aggregation. (A) PMEA accumulation and efflux assay was performed with Abcc4 WT and KO MEFs in the presence of varying concentrations of cilostazol. (B) PMEA export is inhibited by cilostazol comparable with MK571. The accumulation data are represented as the amount of [3H] PMEA (pmol) accumulated per mg of protein. The [3H] PMEA export data are presented as a percentage of total. (C) The impact of cilostazol on the aggregation of Abcc4 WT and KO platelets treated with collagen (P < .05). (D) Model depicting how cilostazol (pink half-circles) inhibits both PDE3 and ABCC4 to elevate cAMP (yellow circles). (E) Representative immunoblot of whole-cell lysates, prepared from Abcc4 WT and KO platelets treated with cilostazol, MK571, or cilostazol+MK571, forskolin (Fsk) 10 μM, and collagen 1 μg/mL for 30 minutes, probed with p-Vasp Ser 157 and total Vasp. (F) Quantification of p-Vasp Ser 157 phosphorylation as presented as ratio of p-Vasp Ser 157:total Vasp.
The phosphodiesterase inhibitor cilostazol is an ABCC4 inhibitor that affects platelet aggregation. (A) PMEA accumulation and efflux assay was performed with Abcc4 WT and KO MEFs in the presence of varying concentrations of cilostazol. (B) PMEA export is inhibited by cilostazol comparable with MK571. The accumulation data are represented as the amount of [3H] PMEA (pmol) accumulated per mg of protein. The [3H] PMEA export data are presented as a percentage of total. (C) The impact of cilostazol on the aggregation of Abcc4 WT and KO platelets treated with collagen (P < .05). (D) Model depicting how cilostazol (pink half-circles) inhibits both PDE3 and ABCC4 to elevate cAMP (yellow circles). (E) Representative immunoblot of whole-cell lysates, prepared from Abcc4 WT and KO platelets treated with cilostazol, MK571, or cilostazol+MK571, forskolin (Fsk) 10 μM, and collagen 1 μg/mL for 30 minutes, probed with p-Vasp Ser 157 and total Vasp. (F) Quantification of p-Vasp Ser 157 phosphorylation as presented as ratio of p-Vasp Ser 157:total Vasp.
ABCC4 and collagen aggregation in human platelets
To investigate the potential role of ABCC4 in collagen-induced aggregation of human platelets, we tested aggregation in the presence and absence of ABCC4 inhibitor, MK571. Incubation of human platelets with MK571 (at 30 µM, a concentration that does not inhibit PDEs; inhibition occurs at concentrations of at least 300 µM26 ) resulted in a modest reduction (7%) in aggregation (Figure 8A). Consistent with reduced aggregation, ABCC4 inhibition produced lower ATP release to ∼0.6 nmol vs WT at 1.28 nmol (Figure 8B). EHNA reduced collagen-mediated aggregation by 15%. The combination of MK571 and EHNA strongly attenuated aggregation by ∼43% compared with platelets treated with collagen alone (Figure 8C-D and supplemental Figure 5), a finding consistent with the greater Vasp phosphorylation at Ser 157 and 239 produced by this combination (Figure 8E).
Blocking ABCC4 function in human platelets coupled with PDE inhibition markedly attenuates aggregation. (A) Blocking of human ABCC4 with MK571 reduces the rate and extent of platelet aggregation. (B) The amount of ATP released from platelets is reduced after ABCC4 inhibition. (C) Human platelet aggregation by collagen is strongly reduced by the combination of the PDE2 inhibitor EHNA and the ABCC4 inhibitor MK571. (D) Lag time (in minutes) in collagen-mediated platelet aggregation after the addition of either the PDE2 inhibitor EHNA, the ABCC4 inhibitor MK571, or both in combination. (E) A representative immunoblot of whole-cell lysates prepared from human platelets treated with EHNA, MK571, or EHNA+MK571 for 30 minutes. Blots were probed with p-Vasp (Ser 157 or Ser 239) and total Vasp antibodies.
Blocking ABCC4 function in human platelets coupled with PDE inhibition markedly attenuates aggregation. (A) Blocking of human ABCC4 with MK571 reduces the rate and extent of platelet aggregation. (B) The amount of ATP released from platelets is reduced after ABCC4 inhibition. (C) Human platelet aggregation by collagen is strongly reduced by the combination of the PDE2 inhibitor EHNA and the ABCC4 inhibitor MK571. (D) Lag time (in minutes) in collagen-mediated platelet aggregation after the addition of either the PDE2 inhibitor EHNA, the ABCC4 inhibitor MK571, or both in combination. (E) A representative immunoblot of whole-cell lysates prepared from human platelets treated with EHNA, MK571, or EHNA+MK571 for 30 minutes. Blots were probed with p-Vasp (Ser 157 or Ser 239) and total Vasp antibodies.
Discussion
Cyclic AMP regulates platelet aggregation response to a variety of agonists.27 Collagen binding to GPVI initiates platelet aggregation, a process antagonized by elevated intracellular cAMP.28 By super-resolution microscopy and plasma membrane analysis, we discovered that human and murine platelets express high amounts of the ABC transporter, ABCC4, almost exclusively at the plasma membrane. Absence of Abcc4 elevates basal cAMP in platelets and hyperelevates intracellular platelet cAMP after prostanoid challenge. This deregulation of cAMP in KO platelets is coupled with reduced plasma membrane expression of GPVI and an impaired binding to collagen. Consistent with impaired aggregation, we showed that Abcc4 absence impaired thrombus formation in the FeCl3 model.11 Further, the impairment in KO platelet aggregation was exaggerated by PDE inhibition, a finding suggesting ABCC4 works in tandem with PDEs to modulate platelet cAMP concentrations. This proposal is supported by our finding that absence of ABCC4, when combined with a PDE inhibitor, produces a dramatic prolongation of bleeding time.
By using a biochemical membrane-labeling technique and super resolution microscopy, we determined that ABCC4 primary location, in human and murine platelets, is the plasma membrane. Notably, Lewandrowski et al, using mass spectrometry, reported that ABCC4 was abundant in platelets.6,29 However, the function of this abundant plasma membrane protein in platelets was unknown. ABCC4 was reported to have a functional role in a key platelet intracellular organelle, the dense-granule.4,5 But, despite multiple publications depicting ABCC4 in dense granules,4,5 no formal demonstration of the actual amount or function of ABCC4 in dense-granules has been documented. These authors proposed that ABCC4 imported ADP into dense-granules after ABCC4-mediated hydrolysis of ATP to ADP.4 However, dense-granule transport of ADP by ABCC4 has not been demonstrated to date. This might be because the studies reporting ABCC4 transport in “dense-granules” was technically flawed. Transport was assessed in a crude mixture of platelet membranes, not pure dense granules.4 This is a problem because our studies demonstrate that the majority of ABCC4 localizes to the platelet plasma membrane. Thus, it is likely that these authors were essentially measuring the transport function of ABCC4 at the plasma membrane in their “crude platelet membrane” preparation. However, if one of the main roles of ABCC4, in platelets, was the export of ADP via dense-granules (or even the plasma membrane), then, in ABCC4’s absence, multifaceted aggregation defects would be anticipated in the KO mouse. Undoubtedly, such defects in aggregation would extend to multiple platelet agonists that use ADP release to accelerate aggregation (eg, collagen, thrombin). However, we show that ABCC4-deficient platelets respond normally to thrombin. In total, these findings indicate ABCC4 is not a general exporter of ADP in platelets and that it is primarily localized to the platelet plasma membrane.
Intriguingly, KO platelets did not have any defect in aggregation with either ADP or thrombin. Both ADP and thrombin modulate intracellular cAMP. After its release, ADP engages the P2Y12 receptor to both restrain cAMP formation via Gi, a negative regulator of adenyl cyclase, and activate phospholipases to accelerate aggregation.30 Based on the WT and KO almost identical dose-response curves for ADP, it is unlikely that ABCC4 contributes to the pathway downstream of P2Y12 receptor activation. In addition, in KO platelets, thrombin-induced aggregation was not affected. Thrombin binds to platelet protease-activated receptors, producing rapid dense granule degranulation and ADP release to propagate aggregation.30 During the intraplatelet thrombin signaling process, before ADP is released, the cAMP-specific PDE3 is activated through phosphorylation.31 Subsequently, PDE3 cleaves cAMP to reduce its level.32 Our studies show that, for thrombin, ABCC4 absence does not modulate aggregation, although we do note that serine phosphorylation (both Ser 157 and Ser 239) of Vasp is reduced in KO platelets. We do not have an explanation for this, but it is possibly related to the basal elevation of cAMP in KO platelets.
The diminished aggregation of KO platelets appears to be specific to collagen. The role of GPVI in collagen mediated aggregation was deciphered by findings from a patient that developed an antibody to GPVI and consequently had reduced GPVI expression coupled with an attenuated collagen aggregation response.16 KO platelets have reduced plasma membrane GPVI, and like patients with decreased GPVI, impaired collagen-mediated aggregation and reduced adherence to collagen. Expression of key molecules mediating the signals downstream of collagen binding, Syk and Plcγ2, are no different in KO platelets. We investigated the role of Vasp, because it is an actin organizer and bona fide regulator of collagen-mediated platelet aggregation because its absence enhances collagen-mediated aggregation.33 On the basis of this, we anticipated that Vasp expression would increase in KO platelets, thereby accounting for their impaired response to collagen. Unexpectedly, Vasp expression was unchanged in KO platelets and the only apparent defect was reduced phosphorylation of Vasp after collagen treatment. However, although enhanced Vasp phosphorylation appears to faithfully reflect cAMP elevation and PKA activation by forskolin, a direct activator of adenylate cyclase, this is less clear for collagen and suggests in some cases that the phosphorylation state of Vasp is modulated by other factors independent of PKA.
Recent studies show that elevated cAMP regulates platelet reactivity to collagen by suppressing GPVI dimerization.34 Elevation of cAMP can also attenuate GPVI signaling by decreasing surface expression of GPVI.35 These results suggest that balancing the intracellular level of cAMP is vital to GPVI localization. Our results underscore this by showing that increased cAMP rapidly reduces GPVI plasma membrane localization. Previous studies have shown that ABCC4 regulates both the intracellular concentration of cAMP as well as cAMP in plasma membrane microdomains.36,37 Further studies are needed to sort out how ABCC4 regulates GPVI plasma membrane localization and the relation with cAMP in the plasma membrane microdomains.
Some mouse knockout models have defective collagen-mediated platelet aggregation, but no differences in bleeding time. For example, the collagen receptor GPVI KO mouse has reduced platelet aggregation in response to collagen (but not other ligands), but bleeding times are comparable with WT.38 Likewise, mice lacking one of the downstream mediators of the collagen Syk also have defective collagen-activated platelet aggregation, but bleeding times comparable with WT mice.39 When we tested bleeding times for KO mice, we discovered they were similar to WT. Because collagen-activated platelet aggregation is attenuated by ABCC4 absence, we tested whether FeCl3-induced vascular endothelial denudation affected platelet mediated vessel occlusion.11 The KO mice had a strongly delayed time to thrombotic occlusion. We extended this to show that some antithrombotic agents (eg, PDEs) may already adventitiously target and inhibit ABCC4. We demonstrated the PDE inhibitor, cilostazol, potently inhibits ABCC4. This might account for some of the noted discrepancies between the low concentrations of PDE inhibitors required to inhibit PDEs in vitro and the unexpectedly high concentrations required for antithrombotic effectiveness in vivo.36 We propose that these high concentrations are required because ABCC4 also needs to be inhibited to elicit the antithrombotic effect.
Our studies suggest that in platelets, ABCC4 cooperates with PDEs in regulating platelet cAMP, and thereby platelet aggregation response in mouse and man. For example, the PDE2 inhibitor EHNA was only capable of dose-dependently attenuating collagen-induced platelet aggregation in KO platelets. These results suggest that, without Abcc4-mediated cAMP export, higher intracellular cAMP concentrations are achieved, leading to reduced platelet aggregation. This concept is extended and reinforced by our findings in the KO mice, which, when combined with PDE2 inhibition (by EHNA), produced a dramatically increased bleeding time. This combination markedly reduced aggregation and enhanced PKA activation, as reflected in the increased Vasp phosphorylation.40 These results suggest that simultaneous inactivation of both ABCC4 and PDEs can potently reduce platelet aggregation and is a potentially important therapeutic strategy.
In total, these studies show that ABCC4 localizes to the plasma membrane and affects collagen-mediated aggregation. Our studies in the KO platelets suggest that sustained inhibition of Abcc4, by producing an elevation in cAMP, attenuates collagen-mediated aggregation. The likely mechanism was caused by a reduction in both the total amount of GPVI and GPVI surface expression. Further, we show that both ABCC4 and PDEs appear to act in tandem to modulate the levels of intraplatelet cAMP, which then modulates collagen-mediated aggregation. This is illustrated by our findings in human platelets, where acute inhibition of ABCC4, coupled with a minimally effective concentration of a PDE inhibitor, markedly attenuated platelet aggregation. These studies have important implications for antithrombotic therapy for individuals with reduced ABCC4 function, because elevated levels of cAMP might reduce GPVI amounts. For example, a nonsynonymous single nucleotide polymorphism in ABCC4, E757K, has strongly reduced function as a result of impaired membrane localization.8 Because this allele occurs frequently in Japanese (18%) and Asians (5%), antiplatelet drug use should be monitored carefully for untoward side effects. Similarly, the concurrent use of drugs that inhibit ABCC4 (eg, ibuprofen, tenofovir, ritonavir9 ) may increase patient risk for untoward bleeding during antithrombotic therapy. The role of impaired ABCC4 function might be especially important for patients receiving antiplatelet drugs that are both PDE inhibitors and ABCC4 substrates (eg, cilostazol).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Cell and Tissue Imaging Center (especially Jamshid Temirov and Jennifer Peters), St. Jude Children’s Research Hospital, for assistance with electron microscopy and immunofluorescence; and Cathy Suggs and Sheri Ring for their phlebotomy expertise.
This work was supported, in whole or in part, by National Institutes of Health (NIH), National Institute of General Medical Sciences grant 2R01GM60904 (J.D.S.), NIH National Cancer Institute grants P30CA21745 and CA21865; and by ALSAC.
Authorship
Contribution: S.B.C., A.P., Y.F., K.T., Y.Z., Y.W., S.F., T.K.G., and T.P. performed experiments; S.B.C., A.P., Y.F., C.J., and J.D.S. analyzed and interpreted data; J.D.S. and C.J. designed the research; S.F. provided critical experimental advice; S.B.C., A.P., and J.D.S. drafted the manuscript; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Schuetz, Pharmaceutical Sciences Department, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, MS 313, Memphis, TN 38105; e-mail: john.schuetz@stjude.org.
References
Author notes
S.B.C. and A.P. contributed equally to this study.

![Figure 5. Abcc4 KO platelets have decreased GPVI expression. (A) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets, loaded at the indicated concentrations, and probed with either GPVI or its binding partner FcRγ. (Β) After densitometry and normalization for loading, the signals were quantified (*P < .05). (C) Immunoblot analysis of total-platelet lysate and surface membrane proteins determined by biotinylation of mouse Abcc4 WT and KO platelets. The transferrin receptor (TFR) was used as a membrane control. (D) After densitometry and normalization by total lysate (Total), the percent on the membrane surface was determined. The error bar represents the standard error of the mean of 3 experiments. (E) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets loaded at the indicated concentrations and probed with Plcγ2, Syk, and actin antibodies. (F) After normalization by actin, the relative amount of Plcγ2 and Syk is shown. (G) Basal cAMP levels in Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05. (H) Ratio of internal vs external cAMP levels post–PGE1 stimulation in the Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05). (I) Immunoblot of platelet-surface membrane proteins obtained after surface biotinylation and avidin-bead pull-down from Abcc4 WT platelets, either untreated or treated with 10 μM of forskolin. Immunoblots were probed with GPVI, as the loading control blot was stained with ponceau S (I, input from total lysate; B, bound to the avidin-bead [ie, surface protein and S in supernatant or cytosol]). (J) Representative Vasp and phospho-Vasp immunoblots in platelet lysates prepared from Abcc4 WT and KO treated with either 1 or 2 μg of collagen, 0.01 U of thrombin, or 10 μM of forskolin. Blots were probed with p-Vasp Ser 157, p-Vasp Ser 239, and an antibody that recognizes total Vasp. Phosphorylation was determined for Ser 157 (K) or Ser 239 (L) as percentage of forskolin (Fsk)-induced phosphorylation. Platelet lysate immunoblot analysis is performed 3 times using pooled PRP-derived platelets from 2 to 4 mice per genotype. The bar in the graph represents 1 standard deviation. Untxt, untreated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/20/10.1182_blood-2014-08-595942/4/m_2307f5.jpeg?Expires=1768165784&Signature=c9EYP28Bv8QSDu4Hdc72-9hUI0vioLLV8yF2Gezm5Kxytvcm6Zz6y3Zu6ZX3kyqW1H53hVCTDoP3eAgGJINKWK38-5cn3l67PNZwC~EvYWdcNLw2NFzWYFXLJb4yPU-0vXJuJPV8PVzQs4SN7f766M4v-RBYa9NGym4Y4077oEnknrjltC6vST3UjKDU1pNUtInVvjGc9Ep5OcQSnoour2-ozFls1TLskkR3w0UhKqwRoIHBxxGauSNpIbVPiRUcMU8J-YF2tX3EJrkEaY0RSpoevdGLl53Lwyrq28Vz1E5UcMqGza4BWy1HgAbgFgIHaXefv5qvIU5dGm48hUWybg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. The phosphodiesterase inhibitor cilostazol is an ABCC4 inhibitor that affects platelet aggregation. (A) PMEA accumulation and efflux assay was performed with Abcc4 WT and KO MEFs in the presence of varying concentrations of cilostazol. (B) PMEA export is inhibited by cilostazol comparable with MK571. The accumulation data are represented as the amount of [3H] PMEA (pmol) accumulated per mg of protein. The [3H] PMEA export data are presented as a percentage of total. (C) The impact of cilostazol on the aggregation of Abcc4 WT and KO platelets treated with collagen (P < .05). (D) Model depicting how cilostazol (pink half-circles) inhibits both PDE3 and ABCC4 to elevate cAMP (yellow circles). (E) Representative immunoblot of whole-cell lysates, prepared from Abcc4 WT and KO platelets treated with cilostazol, MK571, or cilostazol+MK571, forskolin (Fsk) 10 μM, and collagen 1 μg/mL for 30 minutes, probed with p-Vasp Ser 157 and total Vasp. (F) Quantification of p-Vasp Ser 157 phosphorylation as presented as ratio of p-Vasp Ser 157:total Vasp.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/20/10.1182_blood-2014-08-595942/4/m_2307f7.jpeg?Expires=1768165784&Signature=YmmB9ptQCRS9HJ1rqvfjajPbEzGMzLHL~D-OZiw4T7Xd~UffDKhqxqHr~nwuZpfaNMe4RoLAHd0rz2SneN-kAhuia39jYAKe-PihAVKE0VjHrethcE7AL3w7t8fXRBH~uBlmsF~d2mP3Qwp9wHBAvm2LA1OFrxsGZMUA-tZ~50JCGQITwMfOVRZHVmC0Ad1AsfqgJbrVaWIWGO7iLcJSkFKhhksgJMgBu54Rdv~TluOLVZ1ml~fE2Ibf07Pogpmb5rijiz2x5M0aGmRHMc7zSgVj1YvS4sFmDyPWHVbUU9RYTYWz8lKJK6E-XtZWkIH5IdVRaHzuSabmgzsad5MViw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Abcc4 KO platelets have decreased GPVI expression. (A) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets, loaded at the indicated concentrations, and probed with either GPVI or its binding partner FcRγ. (Β) After densitometry and normalization for loading, the signals were quantified (*P < .05). (C) Immunoblot analysis of total-platelet lysate and surface membrane proteins determined by biotinylation of mouse Abcc4 WT and KO platelets. The transferrin receptor (TFR) was used as a membrane control. (D) After densitometry and normalization by total lysate (Total), the percent on the membrane surface was determined. The error bar represents the standard error of the mean of 3 experiments. (E) Immunoblots of whole-cell lysates prepared from Abcc4 WT and KO platelets loaded at the indicated concentrations and probed with Plcγ2, Syk, and actin antibodies. (F) After normalization by actin, the relative amount of Plcγ2 and Syk is shown. (G) Basal cAMP levels in Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05. (H) Ratio of internal vs external cAMP levels post–PGE1 stimulation in the Abcc4 WT and KO platelets measured by competitive enzyme-linked immunosorbent assay (*P < .05). (I) Immunoblot of platelet-surface membrane proteins obtained after surface biotinylation and avidin-bead pull-down from Abcc4 WT platelets, either untreated or treated with 10 μM of forskolin. Immunoblots were probed with GPVI, as the loading control blot was stained with ponceau S (I, input from total lysate; B, bound to the avidin-bead [ie, surface protein and S in supernatant or cytosol]). (J) Representative Vasp and phospho-Vasp immunoblots in platelet lysates prepared from Abcc4 WT and KO treated with either 1 or 2 μg of collagen, 0.01 U of thrombin, or 10 μM of forskolin. Blots were probed with p-Vasp Ser 157, p-Vasp Ser 239, and an antibody that recognizes total Vasp. Phosphorylation was determined for Ser 157 (K) or Ser 239 (L) as percentage of forskolin (Fsk)-induced phosphorylation. Platelet lysate immunoblot analysis is performed 3 times using pooled PRP-derived platelets from 2 to 4 mice per genotype. The bar in the graph represents 1 standard deviation. Untxt, untreated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/20/10.1182_blood-2014-08-595942/4/m_2307f5.jpeg?Expires=1768851954&Signature=uQroHKJywAKdTLo-IQruX3C~8JB64B8li3uDWKybWYY5fugdW04-1veKg5b1UQRdFtVdsfJAeBu1~qxWofBe5O9jDND~iTgVmc79tcl3p3gwimHVvhxpWZ2D02DJIWSoc0K0KDaN31Ue8ZxwLuq-bKDmO6VLiv67ArNhnZ4uphJtUy2aiytFZzDFn6Hb-pPB4ucLzlmgEHZnvOl1R7hJq5rLT1n3YLs~ysPP-l6k3-BepN2A53sUSmYZjkgvOQqebj0HERwDB~4uLtrvJQHQ-o2bwPhrs9y50VR3TPpIJ4rRk2EpDYOtV4Y2jHVX3MvfxG8BosvQUAX04hFrNHXdww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. The phosphodiesterase inhibitor cilostazol is an ABCC4 inhibitor that affects platelet aggregation. (A) PMEA accumulation and efflux assay was performed with Abcc4 WT and KO MEFs in the presence of varying concentrations of cilostazol. (B) PMEA export is inhibited by cilostazol comparable with MK571. The accumulation data are represented as the amount of [3H] PMEA (pmol) accumulated per mg of protein. The [3H] PMEA export data are presented as a percentage of total. (C) The impact of cilostazol on the aggregation of Abcc4 WT and KO platelets treated with collagen (P < .05). (D) Model depicting how cilostazol (pink half-circles) inhibits both PDE3 and ABCC4 to elevate cAMP (yellow circles). (E) Representative immunoblot of whole-cell lysates, prepared from Abcc4 WT and KO platelets treated with cilostazol, MK571, or cilostazol+MK571, forskolin (Fsk) 10 μM, and collagen 1 μg/mL for 30 minutes, probed with p-Vasp Ser 157 and total Vasp. (F) Quantification of p-Vasp Ser 157 phosphorylation as presented as ratio of p-Vasp Ser 157:total Vasp.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/20/10.1182_blood-2014-08-595942/4/m_2307f7.jpeg?Expires=1768851954&Signature=zoXouI5zmnjohfW8khtxlOy9xyMlk3PSQ37gEvLxkn~NCb3FTQ-uHozNF2CElDW2ruH2F2xk9Ak1TpFKa-saG8p9RQmTeQ~XfB9VQSQ4EehHmU~m8SiXlffcga8pfoRMrGFuXjkSPh52-vC5~WtmbirGRXaP67MuCPq1feKCF~-Rw31Lp2TWeLr6z~sUh712~Ieuu9Aj-oj2LVZVnE7fxtd-3Pfeb5n-pRe7SWLWrxXVuD~a9GToGYRTA5V385R-SzoYjx651qmBpd~lhgkpc5F3HWKiMsTB3sYK7DvqCUKViAYV0oWewuwYm2QJwyKC0JMHOJbeXKzbnjankM-4KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)