In this issue of Blood, Hur et al1 hit the jackpot with their discovery that plasmin inactivates activated blood coagulation factor XIIIa (FXIIIa), but not zymogen factor XIII (FXIII), and that FXIIIa is inactivated during clot lysis, but not during clot formation. Their study resolves years of conflicting results regarding the inactivation of FXIIIa by plasmin.
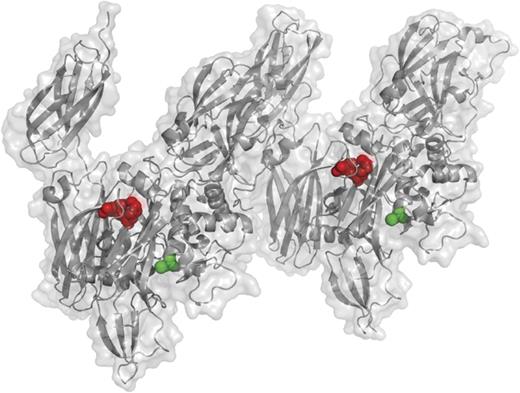
This projection, based on a previously solved structure of the activated FXIIIa A2 dimer,8 shows the K468-Q469 plasmin cleavage site in red and its proximity to the active-site cysteine in green. This site is cleaved only in activated FXIIIa, not FXIII zymogen, and this cleavage event inactivates FXIIIa. See Figure 2B in the article by Hur et al that begins on page 2329.
This projection, based on a previously solved structure of the activated FXIIIa A2 dimer,8 shows the K468-Q469 plasmin cleavage site in red and its proximity to the active-site cysteine in green. This site is cleaved only in activated FXIIIa, not FXIII zymogen, and this cleavage event inactivates FXIIIa. See Figure 2B in the article by Hur et al that begins on page 2329.
FXIII, known previously as “fibrin stabilizing factor” and “Laki-Lóránd factor,” is unique among all the coagulation factors.2 In addition to its distinction of having the last Roman numeral of the numbered coagulation factors and being the last to be activated, it is the only zymogen transglutaminase in the blood coagulation cascade. Most of the other named coagulation factors are either serine protease zymogens or zymogen cofactors, and none of them have transglutaminase activity. FXIII circulates in 2 forms, a plasma form and a platelet form.3 The plasma form is a heterotetramer of 320 kDa that consists of 2 83-kDa A subunits and 2 77-kDa B subunits that circulates at concentrations of about 10 μg/mL. The A subunits are the transglutaminase zymogens. In contrast, the platelet form of FXIII consists only of dimeric A subunits and is stored in platelet α granules until the platelets are activated.
FXIII zymogen is activated via a single proteolytic cleavage by thrombin at the R37-G38 bond, releasing a 4-kDa activation peptide and converting FXIII into FXIIIa.4 In the plasma form, the B subunits subsequently dissociate, resulting in an activated A2 dimer, termed A2*. In platelet FXIII, the A2 dimer is activated by thrombin after its release from the platelet. In both forms, the active A2* dimer covalently crosslinks specific substrate proteins, particularly fibrin.5 This crosslinking is achieved by an unusual reaction in which glutamine side chains in substrate proteins are crosslinked with closely apposed lysine side chains that have the appropriate spatial geometry.2 The glutamine and lysine side chains can either be in the same protein or in separate proteins, resulting in covalent isopeptide bonds consisting of γ-glutamyl-ε-lysine crosslinks. For example, fibrin strands can be covalently crosslinked together, resulting in fibrinolytic resistance. In addition, the fibrinolytic inhibitor α2-antiplasmin can be covalently crosslinked to fibrin, which also results in fibrinolytic resistance.6 The well-known D-dimers that are used clinically to assess a variety of coagulation pathophysiologies have their origin in the FXIIIa-mediated crosslinking of fibrin strands. FXIII is required for optimal fibrin strength and fibrinolytic resistance; deficiencies can result in a lifelong bleeding diathesis, impaired wound healing, and chronic miscarriage.3
The regulation of activated FXIIIa has remained an enigma for many years.7 Unlike the activated serine protease coagulation factors, which are typically inactivated by serpins such as antithrombin, there are no accessory proteins that combine with FXIIIa to inactivate its active site cysteine. Previous studies had suggested that plasmin, which is activated during fibrinolysis, inactivates FXIIIa by proteolysis, somewhat reminiscent of factors Va and VIIIa inactivation by activated protein C. The study by Hur et al1 clarifies many aspects of this proteolytic inactivation of FXIIIa by plasmin. Their findings show that plasmin cleaves the K468-Q469 bond in active FXIIIa, but not in the FXIII zymogen (see figure8 ). This cleavage event inactivates FXIIIa, preventing further crosslinking of its substrates. Additional cleavage sites were also observed, but their resulting peptide fragments were detected 8 to 38 times less frequently than the K468-Q469 cleavage. These results have clinical implications in the use of tissue plasminogen activator (tPA) for thrombolysis during heart attack and stroke. The authors propose that inactivation of FXIIIa by plasmin due to tPA administration may contribute to the hemorrhagic side effects of tPA. It is tempting to speculate that administration of FXIII9 might decrease tPA-induced hemorrhage by restoring FXIIIa that has been inactivated by plasmin, but further studies are necessary to test this hypothesis.
Conflict-of-interest disclosure: Oregon Health & Science University and D.H.F. have a significant interest in Gamma Therapeutics, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict of interest has been reviewed and managed by Oregon Health & Science University.