In this issue of Blood, Gill et al describe the results of the first phase 3 clinical trial evaluating recombinant von Willebrand factor (rVWF) for the treatment of hemorrhagic events in all patients with von Willebrand disease (VWD).1
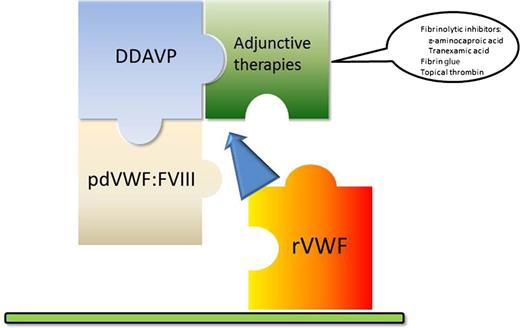
Pieces of the von Willebrand disease treatment puzzle. The current available options for the treatment of VWD have remained essentially unchanged for years. While the use of plasma-derived VWF (pdVWF:FVIII) was a major advance over plasma or cryoprecipitate, it is still associated with allergic reactions and the potential to transmit infectious agents. Desmopressin (DDAVP) remains an effective treatment for mild bleeds or procedures, and has the advantage of home treatment. Its use is limited to just a few doses. Inhibitors of fibrinolysis are useful for bleeds in mucosal tissue, but play a limited role. It is anticipated that rVWF will offer a more complete treatment paradigm to patients with VWD.
Pieces of the von Willebrand disease treatment puzzle. The current available options for the treatment of VWD have remained essentially unchanged for years. While the use of plasma-derived VWF (pdVWF:FVIII) was a major advance over plasma or cryoprecipitate, it is still associated with allergic reactions and the potential to transmit infectious agents. Desmopressin (DDAVP) remains an effective treatment for mild bleeds or procedures, and has the advantage of home treatment. Its use is limited to just a few doses. Inhibitors of fibrinolysis are useful for bleeds in mucosal tissue, but play a limited role. It is anticipated that rVWF will offer a more complete treatment paradigm to patients with VWD.
VWD is a congenital bleeding disorder, with prevalence reported to be 1% in the general population2 and as high as 1.3% in the pediatric setting,3 and it is considered to be the most common congenital hemorrhagic disease.4 It is categorized by either a quantitative (types 1 and 3) or qualitative (types 2A, 2B, 2M, and 2N) defect in von Willebrand factor (VWF), which can manifest as excessive posttraumatic hemorrhage to life-threatening bleeding. The symptoms of VWD are commonly associated with mucocutaneous bleeding, such as epistaxis, gastrointestinal hemorrhage, and menorrhagia. Hemarthrosis, usually seen in hemophilia, is typically limited to those with a near absence of VWF, namely type 3 VWD.5
The current landscape for the treatment of VWD is limited to: (1) desmopressin, (2) plasma-derived VWF:factor VIII (FVIII) concentrates, and (3) adjunctive therapies such as fibrinolytic inhibitors (see figure). The use of desmopressin is usually considered effective in type 1 VWD for minor bleeding episodes or minor procedures, and it is recommended to evaluate a patient’s reaction to desmopressin because many may not achieve a meaningful response.6 Potential adverse effects include a loss of response (ie, tachyphylaxis) with repeated dosing, hyponatremia, and the risk of thrombosis in patients with underlying hypercoagulability.7,8
For significant hemorrhagic events or major procedures, replacement with factor concentrates is recommended.4 Although the use of recombinant factor replacement has become the norm for the treatment of FVIII and FIX deficiency, the only 2 preparations that are Food and Drug Administration–approved for use in VWD (Humate-P, CSL Behring; Alphanate, Grifols) are limited to plasma-derived products that contain varying amounts of FVIII. These agents are effective for the prevention and treatment of bleeding in VWD, yet adverse events such as allergic reactions and thrombosis have been reported.9,10 In addition, because these agents are derived from donor plasma, the risk for the transmission of viral or prion disease(s) remains ever present. As such, the time has long since passed for the next step forward in the treatment of VWD, in terms of both improved efficacy and safety.
Enter the development and evaluation of rVWF in a phase 3 clinical trial presented by Gill et al.1
Thirty-seven adults with all VWD subtypes (except 2B and 2M) were enrolled in 1 of 4 arms to evaluate the pharmacokinetics of rVWF with or without supplemental rFVIII, in addition to the use of rVWF for the treatment of bleeding episodes. Because the rVWF is without any coprecipitating FVIII, an initial dose of rFVIII (Advate, Baxalta) was given if needed, and once the study subjects’ own FVIII levels rose, it was believed no additional dosing was necessary. Even without the additional rFVIII, when rVWF was given alone, the authors observed that the study subjects’ endogenous FVIII levels rose to >40% within 6 hours. This is an important aspect of the use of rVWF, because if repeated dosing is needed, increasing FVIII levels are avoided, which would reduce the risk for thrombotic complications. It was also noted that the half-life of the infused rVWF approached 22 hours—this is interpreted by the authors to be caused in part by the presence of the ultrahigh-molecular-weight multimers present in the rVWF, which are not present in the same amounts in the currently available plasma-derived preparations of VWF.
Probably the most exciting aspect of this trial is the hemostatic efficacy of the rVWF compound. This is effectively summarized by the observation that the majority of bleeds were effectively treated by a single dose of rVWF (81.8%), major bleeding with a median of 2 infusions, and only 1 study subject requiring 4 infusions. Just as important are the safety parameters, which showed that the rVWF was well-tolerated, an immune response against the product was not documented, and no thrombotic events were recorded. Long-term safety data and outcomes are lacking, particularly compared with the currently available recombinant factors. Once rVWF is approved for clinical use, continued surveillance will be needed.
The potential of rVWF is characterized by the absence of FVIII in the treatment product, thereby minimizing the risk for thrombosis, near absent risk for the transmission of infectious agents, reduced chances for allergic/anaphylactoid reactions from contaminating plasma products, noted efficacy of promoting hemostasis, and the initial safety profile. As the use of recombinant factor replacements has become available for the treatment of FVII, FVIII, and FIX deficiencies, modern-day treatment of VWD has lagged behind. Given the limitations of the therapies currently available for VWD, it is anticipated that the use of rVWF will provide the next step forward in the care of patients with VWD.
Conflict-of-interest disclosure: The author declares no competing financial interests.