In this issue of Blood, Bernard et al provide evidence that ibrutinib, the orally administered inhibitor of Bruton tyrosine kinase (BTK), crosses the blood-brain barrier and has activity against mantle cell lymphoma (MCL) in the central nervous system (CNS).1
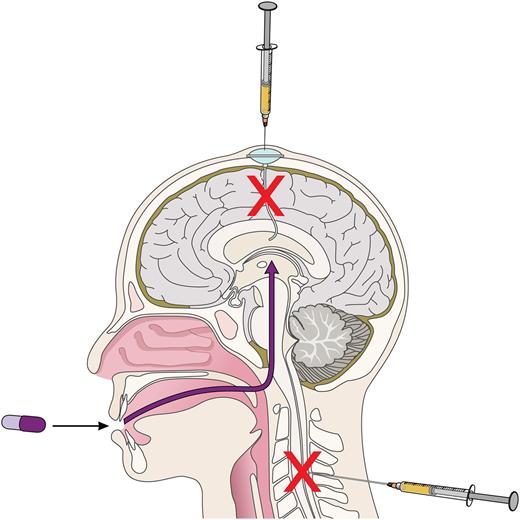
Different routes of CNS-directed therapies in MCL. Among patients with MCL, the average survival following development of CNS involvement is typically <6 months, despite treatment with intravenous and intrathecal chemotherapy. Bernard et al describe 3 cases of CNS MCL with ongoing remissions at 2 months, 9 months, and 1 year. Professional illustration by Patrick Lane, ScEYEnce Studios.
Different routes of CNS-directed therapies in MCL. Among patients with MCL, the average survival following development of CNS involvement is typically <6 months, despite treatment with intravenous and intrathecal chemotherapy. Bernard et al describe 3 cases of CNS MCL with ongoing remissions at 2 months, 9 months, and 1 year. Professional illustration by Patrick Lane, ScEYEnce Studios.
Over the last decade, several groups have evaluated the incidence and risk factors for CNS involvement by MCL.2-5 Like diffuse large B-cell lymphoma (DLBCL), roughly 5% of patients with MCL will develop CNS disease. Like DLBCL, patients with the most aggressive MCL biology (eg, blastoid histology, high lactate dehydrogenase, and high Ki67) are most likely to experience CNS relapse, as many as 25% in some reports. Like DLBCL, there is limited evidence in MCL that the choice of primary therapy impacts the incidence of CNS relapse. The use of agents with potential activity against CNS lymphoma, including rituximab, high-dose cytarabine, methotrexate, and autologous stem cell transplantation, was not associated with a reduced risk of CNS involvement in 3 retrospective series, although the dosing of methotrexate may have been suboptimal for CNS penetration, and the use of prophylactic intrathecal therapy was not reported.2,4,5 Finally, like DLBCL, the survival of people that experience CNS progression of MCL is typically poor despite subsequent administration of CNS-directed intravenous or intrathecal therapies (see figure). However, MCL and DLBCL are biologically distinct entities, and there are important differences with respect to CNS involvement. Unlike DLBCL, which tends to relapse in the brain parenchyma within the first 6 months of diagnosis, the average time to progression of MCL in the CNS, often with leptomeningeal disease, is typically >1 year from diagnosis and frequently occurs after multiple prior lines of therapy. Unlike DLBCL, regardless of location, MCL is not curable.
Recent reports of oral agents with newly discovered activity against CNS lymphomas provide great news for patients. There have been multiple reports, including at least 1 prospective clinical trial, demonstrating the activity of oral lenalidomide for primary CNS lymphoma.6,7 Although the report from Bernard et al consists of just 3 patients, the data demonstrate that ibrutinib may have durable clinical benefit in some patients with CNS MCL. That 2 of the 3 patients had a Ki67 of ≥60% makes the report even more remarkable, given the suggestion that ibrutinib is less effective in more proliferative tumors.8 It raises the question of whether ibrutinib might have a differential effect on tumor cells in the CNS compared with the lymph node microenvironment. It is likely that we will see additional reports of similar activity in the future, but given publication bias toward positive results, it is unlikely that we will gain a true idea of the activity of ibrutinib against CNS MCL outside of a clinical trial like the National Cancer Institute phase 2 trial of immunochemotherapy plus ibrutinib in primary CNS lymphoma.9 Prospective trials will also be required to better define the pharmacokinetics of ibrutinib in the cerebrospinal fluid (CSF), including CNS penetration, as the blood-brain barrier evolves during tumor resolution. Still larger data sets might be required to interpret the pharmacokinetics relative to clinical response. It is worth noting that in the initial phase 1 trial with ibrutinib, doses as low as 1.25 mg/kg achieved serum concentrations that exceeded the IC50 (and the CSF concentration reported by Bernard et al), whereas 2.5 mg/kg was sufficient to achieve maximal BTK occupancy 4 hours after administration, and the standard 560 mg daily dose of ibrutinib approved for MCL is threefold higher.
The Bernard et al data raise the question of whether we might see new strategies to reduce the occurrence of MCL progression within the CNS among high-risk patients similar to strategies used in DLBCL. It may be more challenging to answer questions about CNS prophylaxis than it is to demonstrate activity of an agent against CNS MCL. The mere concept that front-line therapy might impact CNS progression is challenging to grasp given that CNS progression is frequently a late event. Unfortunately, clinical trials rarely collect data on sites of relapse or outcomes of subsequent therapies, and this information is challenging to collect retrospectively. Moreover, better front-line strategies may paradoxically give the appearance of an increase in the incidence of CNS involvement if more patients live longer. Similarly, because MCL is likely as incurable in the CNS as it is elsewhere, it is possible that different front-line strategies will not actually impact the incidence but rather the timing of CNS progression (still a worthy goal). Reports of overall incidence may be less informative than time-to-event statistics. Finally, because the proportion of patients at highest risk for CNS progression is quite low in most clinical trials, individual studies may have limited power to demonstrate a difference and meta-analyses may be required.
Regardless of the challenges involved in data interpretation, the fact that there are now well-tolerated agents with a hint of activity against CNS MCL is a step forward. Reports such as this one by Bernard et al demonstrate the ongoing importance of clinical observation.
Conflict-of-interest disclosure: P.M. has received honoraria from Janssen and Genentech and has served as a consultant for Janssen, Celgene, Acerta, Bayer, Gilead, Genentech, and Novartis.