Abstract
“Langerhans cell histiocytosis” (LCH) describes a spectrum of clinical presentations ranging from a single bone lesion or trivial skin rash to an explosive disseminated disease. Regardless of clinical severity, LCH lesions share the common histology of CD1a+/CD207+ dendritic cells with characteristic morphology among an inflammatory infiltrate. Despite historical uncertainty defining LCH as inflammatory vs neoplastic and incomplete understanding of mechanisms of pathogenesis, clinical outcomes have improved markedly over the past decades through cooperative randomized clinical trials based on empiric therapeutic strategies. Significant advances include recognition of high- and low-risk clinical groups defined by hematopoietic and/or hepatic involvement, and of the importance of optimal intensity and of duration of chemotherapy. Nevertheless, mortality of high-risk patients, disease recurrence, lack of robustly tested salvage strategies, and significant disease morbidity of both high- and low-risk patients remain challenges. Recent discovery of recurrent somatic mutations in mitogen-activated protein kinase pathway genes at critical stages of myeloid hematopoietic differentiation in LCH patients supports redefinition of the disease as a myeloproliferative disorder and provides opportunities to develop novel approaches to diagnosis and therapy.
Clinical scenarios
Case 1
A newborn has a scaly rash diagnosed as “cradle cap.” Due to persistence despite topical therapy for 2 months, a skin biopsy is diagnostic of Langerhans cell (LC) histiocytosis (LCH). Evaluation for other sites of disease reveals he has “skin-only” LCH. By 4 months, the rash begins to resolve without therapy and by 1 year it disappears.
Case 2
A newborn has a scaly rash diagnosed as “cradle cap.” The rash persists, but no investigations are done until 22 months when she develops dyspnea and begins waking up at night crying for a bottle. Chest radiograph shows a diffuse interstitial pattern and computed tomography (CT) scan reveals large pulmonary cysts and nodules. Magnetic resonance imaging (MRI) of her pituitary shows absence of the posterior bright spot and an enlarged stalk. Positron emission tomography (PET) scan reveals abnormal uptake in mastoid, liver, spleen, and lungs. Biopsy of the mastoid is consistent with LCH. Bone marrow biopsy does not demonstrate CD1a+/CD207+ histiocytes, but quantitative polymerase chain reaction (qPCR) estimates 1% of marrow aspirate cells carry the BRAF-V600E mutation. She is treated with vinblastine/prednisone for 12 weeks with no response. She then receives cytarabine for 2 months with little change in the PET scan and her rash persists. Finally, she is treated with clofarabine and achieves a complete remission.
Case 3
A 55-year-old woman with a 30-year history of 2-pack-per-day cigarette smoking presents with acute chest pain and dyspnea. Chest radiograph reveals a right-sided pneumothorax with multiple cysts throughout the lungs, and CT scan demonstrates innumerable parenchymal lesions. Lung biopsy reveals CD1a+/CD207+ cells, consistent with LCH. PET scan and other evaluations do not identify other sites of disease. The pneumothorax resolves following video-assisted thoracoscopic surgery and the lung lesions regress after 6 months of smoking cessation.
Case 4
A 35-year-old woman has a 5-year history of ulcerative vaginal rashes resulting in chronic pain. Various topical ointments and oral antibiotics were ineffective. In the past 12 months, she has developed extraordinary thirst (3 gallons of water daily). Repeated tests for glycosuria and hyperglycemia are negative. She has ataxia, tremors, and severe memory deficits. MRI is performed and identifies a large enhancing hypothalamic lesion, pituitary stalk thickening, absence of the posterior pituitary bright spot, and T2 and fluid-attenuated inversion recovery (FLAIR) enhancement in the cerebellar white matter. Biopsy of a vaginal papule is consistent with LCH. Treatment with cytarabine is initiated. Six months later, the brain MRI normalizes and vaginal lesions resolve.
Introduction
As evidenced by these cases, LCH encompasses a broad spectrum of clinical manifestations in children and adults, ranging from self-limited lesions to life-threatening disseminated disease, with the common feature of inflammatory lesions that include CD1a+/CD207+ dendritic cells (DCs) (Figure 1). LCH remains a major clinical problem with incidence of ∼5 cases per million children (similar to pediatric Hodgkin lymphoma)1-3 and, although probably incompletely recognized and reported, an estimated 1 to 2 cases per million adults.4 Outcomes have improved substantially for patients with disseminated high-risk LCH. However, over 10% still die of their disease, and significant numbers of patients experience reactivations and long-term morbidity. Progress has been challenged by incomplete understanding of pathogenesis and uncertain categorization of LCH as an inflammatory vs a neoplastic disorder. Recent discoveries of recurrent activating mutations in mitogen-activated protein kinase (MAPK) pathway genes provide opportunities to test novel rational therapeutic strategies.
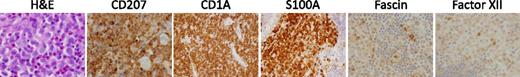
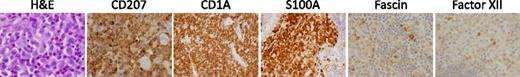
Histology of LCH lesions. This figure depicts a typical LCH lesion with characteristic histology. Pathologic “histiocytes” with reniform nuclei and eosinophilic cytoplasm are scattered among an infiltrate of lymphocytes, eosinophils, and macrophages. The LCH cells react to immunostains for CD207, CD1a, and S100, but not for fascin or factor XIIIa. This biopsy could be from any child or adult with low- or high-risk LCH. H&E, hematoxylin and eosin. Images courtesy of John Hicks, Baylor College of Medicine.
Histology of LCH lesions. This figure depicts a typical LCH lesion with characteristic histology. Pathologic “histiocytes” with reniform nuclei and eosinophilic cytoplasm are scattered among an infiltrate of lymphocytes, eosinophils, and macrophages. The LCH cells react to immunostains for CD207, CD1a, and S100, but not for fascin or factor XIIIa. This biopsy could be from any child or adult with low- or high-risk LCH. H&E, hematoxylin and eosin. Images courtesy of John Hicks, Baylor College of Medicine.
The vast majority of data in LCH is generated from randomized pediatric clinical trials and case series. The ability to extrapolate the pediatric experience with risk assessment and therapy to adults remains uncertain. In a dedicated section, we highlight special considerations for adult patients.
A brief history
LCH first appeared in the medical literature around 1900 with reports of children with skin lesions, lytic bone lesions, and diabetes insipidus (DI), classified as Hand-Schüller-Christian disease. Reports also emerged of infants with disseminated inflammatory lesions including liver, spleen, and bone marrow, classified as Letterer-Siwe disease.5,6 In the 1950s, Lichtenstein noted the common histologic appearance of lesions from “eosinophilic granulomas,” Hand-Schüller-Christian disease, and Letterer-Siwe disease, hypothesized a common cell of origin, and proposed the name “Histiocytosis X,” with “X” indicating the uncertain origin.7 In the 1970s, Nezelof discovered the presence of Birbeck granules, a cytoplasmic structure ultimately associated with langerin (CD207), in the lesional LCH cells (Figure 1). Because Birbeck granules had only been identified in epidermal LCs, Nezelof hypothesized that “Histiocytosis X” arises from the epidermal LC lineage, and the disease has since been named “Langerhans cell histiocytosis.”8
New biology as a myeloproliferative neoplasia
Alternative origins of LCH
From the 1970s until 2010, debates on LCH pathogenesis centered around the nature of LCH as a neoplastic transformation vs dysregulated immune activation of the epidermal LC.9 However, the origin of LCH cells from the epidermal LC lineage has recently been challenged. Langerin expression is more widespread than previously appreciated: blood-derived CD207+ DCs distinct from epidermal LCs are detected in lymphoid and nonlymphoid organs where LCH lesions arise, making alternative origins of LCH cells plausible,10-13 and gene expression profiling is more consistent with the LCH cell being a myeloid precursor than a differentiated epidermal LC.14
Somatic mutations in MAPK genes
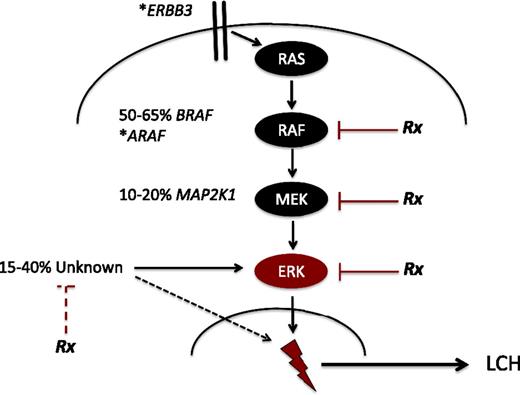
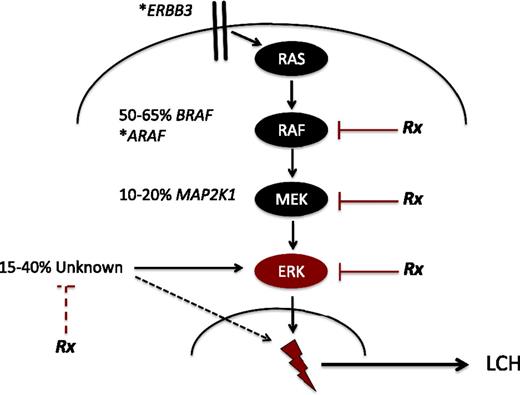
A breakthrough in understanding of LCH pathogenesis came with the discovery15 and validation16-19 of recurrent BRAF-V600E mutations in over 50% of LCH lesions. BRAF, a central kinase of the MAPK pathway, regulates critical cellular functions (Figure 2). The BRAF-V600E mutation induces constitutive activation of downstream MAPK/ERK kinase (MEK) and extracellular signal-regulated kinase (ERK) proteins. Whole-exome sequencing and targeted sequencing recently identified recurrent mutations in MAP2K1, encoding MEK1, in 33% to 50% of LCH lesions in which BRAF is not mutated.20,21 Additional cases of other mutations in MAPK genes have also been reported, including ARAF and ERBB3.21,22 The importance of MAPK signaling in LCH pathogenesis is further supported by ERK phosphorylation in all LCH cells reported to date.15,21
Recurrent activating MAPK mutations in LCH. Known recurrent mutations in LCH are depicted. The majority of patients have mutually exclusive activating mutations in BRAF (BRAF-V600E) or MAP2K1. Individual cases of mutations in ARAF and ERBB3 have also been reported (*). In 15% to 40% of LCH lesions, no MAPK somatic mutations are identified. However, early series suggest ERK is activated in all cases of LCH. The dashed black line represents mechanisms of ERK activation outside of the MAPK pathway that remain to be defined. “Rx” represents steps in ERK activation that may be therapeutic targets. The red dashed line represents potential to target mechanisms outside of the MAPK pathway (eg, phosphatidylinositol 3-kinase/AKT) that may inhibit ERK activation by alternative pathways. The lightning bolt represents mechanisms downstream of ERK activation, that remain to be defined, that lead to LCH pathogenesis.
Recurrent activating MAPK mutations in LCH. Known recurrent mutations in LCH are depicted. The majority of patients have mutually exclusive activating mutations in BRAF (BRAF-V600E) or MAP2K1. Individual cases of mutations in ARAF and ERBB3 have also been reported (*). In 15% to 40% of LCH lesions, no MAPK somatic mutations are identified. However, early series suggest ERK is activated in all cases of LCH. The dashed black line represents mechanisms of ERK activation outside of the MAPK pathway that remain to be defined. “Rx” represents steps in ERK activation that may be therapeutic targets. The red dashed line represents potential to target mechanisms outside of the MAPK pathway (eg, phosphatidylinositol 3-kinase/AKT) that may inhibit ERK activation by alternative pathways. The lightning bolt represents mechanisms downstream of ERK activation, that remain to be defined, that lead to LCH pathogenesis.
LCH as a myeloid neoplasia
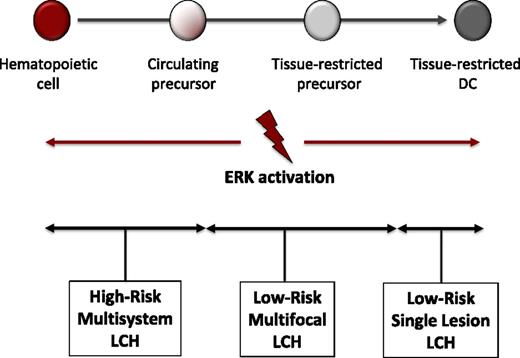
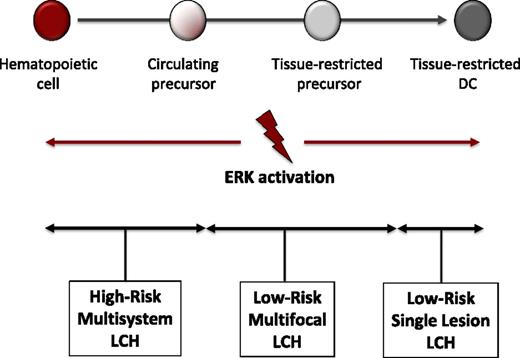
Although BRAF-V600E arises in many malignancies, it also is found in benign growths, such as melanocytic nevi.23,24 The significance of hyperactive ERK in driving LCH pathogenesis is supported by the ability of enforced expression of conditional BRAF-V600E driven by CD11c-Cre to recapitulate a LCH-like phenotype in mice, whereas a more attenuated phenotype was observed when Cre was under control of a langerin promoter.16 Furthermore, BRAF-V600E was identified in hematopoietic cells in bone marrow of patients with high-risk LCH. Based on these data, we propose the misguided myeloid DC model of LCH pathogenesis where the state of cell differentiation in which pathologic ERK activation arises determines the clinical extent of LCH (Figure 3). The finding of mutations with potential to drive pathogenesis of LCH in hematopoietic stem cells and myeloid precursor cells supports classification of LCH as a “myeloproliferative neoplasm.”25 The inflammatory infiltrate also likely contributes to aspects of pathogenesis. LCH may therefore constitute an “inflammatory myeloid neoplasia.”9
Misguided myeloid DC model of LCH pathogenesis. According to this model, the stage of differentiation in which pathologic ERK activation arises determines the clinical manifestations of LCH. In this model, activating mutations in hematopoietic stem cells or undifferentiated myeloid DC precursors result in multifocal high-risk disease, whereas mutations in tissue-restricted precursors results in multifocal low-risk disease, and mutations in more differentiated tissue-restricted precursor cells result in a single lesion. The CD207+ cells in LCH lesions represent differentiated myeloid cells with indistinguishable phenotype regardless of cell of origin that presumably recruit and activate other inflammatory cells.
Misguided myeloid DC model of LCH pathogenesis. According to this model, the stage of differentiation in which pathologic ERK activation arises determines the clinical manifestations of LCH. In this model, activating mutations in hematopoietic stem cells or undifferentiated myeloid DC precursors result in multifocal high-risk disease, whereas mutations in tissue-restricted precursors results in multifocal low-risk disease, and mutations in more differentiated tissue-restricted precursor cells result in a single lesion. The CD207+ cells in LCH lesions represent differentiated myeloid cells with indistinguishable phenotype regardless of cell of origin that presumably recruit and activate other inflammatory cells.
Clinical presentations and evaluation
Diagnosis
As illustrated by the introductory cases, LCH is a challenging diagnosis due to the spectrum of clinical manifestations and overlap with more common conditions. In 1 pediatric series, median time to diagnosis from initial symptoms exceeded 3 months.26 Anecdotally, we have treated adults who had active disease for decades before being diagnosed. The critical element for diagnosis is biopsy with characteristic histiocytes with surface expression of CD207 (langerin) and CD1a. Excisional biopsy is optimal to obtain a sample with intact architecture. Although normal skin and lymph node biopsies include scattered physiologic LCs, abnormal clusters of CD1a+/CD207+ histiocytes define LCH. Comprehensive histologic evaluation including antibodies against CD163, fascin, and factor XIII is helpful to identify mixed histiocytic lesions (eg, juvenile xanthogranuloma/LCH or Erdheim-Chester [ECD]/LCH).
Extent of disease
Initial screening tests followed by directed testing define the extent of disease.27 Studies for newly diagnosed LCH patients include skull series, skeletal survey, chest radiograph, complete blood count, liver enzymes including aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transferase, and assessment of synthetic function. PET scans are effective to screen for lesions.28 Ultrasound or MRI may identify lesions in patients with suspected liver involvement. CT scanning is useful to assess lesions in the orbit, mastoid, sphenoid, and temporal bones. MRI is effective in evaluating lesions in brain, pituitary, vertebrae, spinal cord, and pelvis.28 We perform bone marrow biopsy and aspirate on patients younger than 2 years old or any patients with cytopenias, and endoscopy with biopsy on patients with evidence of malabsorption (Table 1).
Risk stratification
Validated risk stratification criteria for children include sites of disease and response to initial therapy. Patients with lesions in “risk organs” including bone marrow, spleen, or liver have significantly higher risk of mortality than patients with lesions limited to “nonrisk” sites. Risk stratification for LCH is based on analysis of outcomes of prospective pediatric trials, and significance of “risk sites” remains uncertain in adults. Patients with high-risk LCH have survival of almost 90%, but outcomes are significantly worse if the disease progresses during the first 12 weeks of therapy.29 We and others have found that the BRAF-V600E mutation is not associated with clinical variables including risk category or survival,15,16,18 but our longitudinal outcome data16 suggest BRAF-V600E may be associated with a twofold increase in risk of treatment failure or reactivation. Prospective trials are needed to validate the impact of specific somatic mutations on clinical outcomes.
Evaluating disease activity and response to therapy
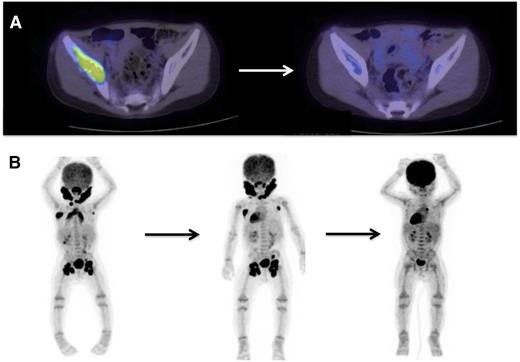
PET scan is effective in evaluating response to treatment of most lesions (Figure 4) except vertebral lesions, which may be better visualized by MRI that will capture changes in soft tissue or enhancement of the bone.28 Vertebral CT scans may add information on bone response to therapy and CT scans are also optimal for following bony lesions of the skull, whereas MRI is most effective for parenchymal brain and pituitary lesions. Bone healing may lag resolution of other lesions (Figures 5-6). CT scanning is also an optimal study to evaluate lung lesions, though cysts may remain as permanent consequences following resolution of nodular parenchymal lung lesions (Figure 7). Clinical examination is sufficient follow-up for proven skin-limited disease, and patients with single bone lesions may be followed by clinical examination, radiograph, or MRI to minimize radiation exposure.
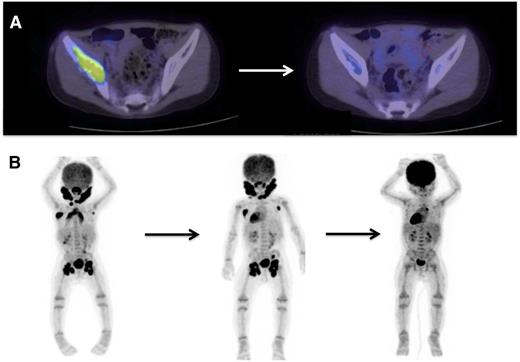
PET/CT scans are effective to stage disease and to evaluate response to therapy in LCH. Examples of 18-fluoro-deoxyglucose–PET scans with CT identifying response to therapy. (A) A patient with multifocal bone disease with interval improvement of pelvic lesion and decreased PET avidity. (B) A patient with multifocal lymph node disease (cervical, axillary, inguinal) who initially failed to respond to cytarabine therapy, then had significant response to 2 cycles of clofarabine.
PET/CT scans are effective to stage disease and to evaluate response to therapy in LCH. Examples of 18-fluoro-deoxyglucose–PET scans with CT identifying response to therapy. (A) A patient with multifocal bone disease with interval improvement of pelvic lesion and decreased PET avidity. (B) A patient with multifocal lymph node disease (cervical, axillary, inguinal) who initially failed to respond to cytarabine therapy, then had significant response to 2 cycles of clofarabine.
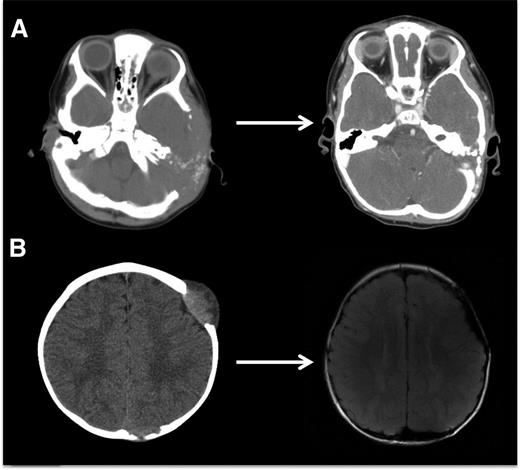
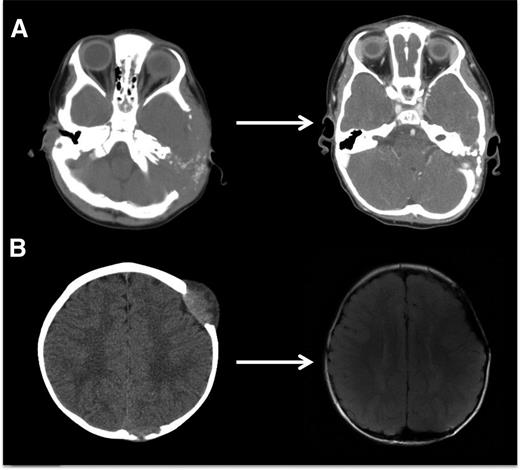
LCH bone lesions may remodel if margins remain intact. These cases highlight the potential for even very large bone lesions to remodel following disease resolution. (A) Skull CT scans before and after chemotherapy in a patient with multifocal bone LCH. Remodeling following systemic chemotherapy nearly normalizes bone structure in a patient with significant skull lesions. This patient did not have any curettage or excisional surgery in the skull. (B) Brain MRI in a patient with multifocal bone LCH before and after complete excision with placement of mesh grafts. Complete excision of LCH lesion with margins into healthy bone inhibits potential for remodeling. Following resections and successful chemotherapy, skull defects persist.
LCH bone lesions may remodel if margins remain intact. These cases highlight the potential for even very large bone lesions to remodel following disease resolution. (A) Skull CT scans before and after chemotherapy in a patient with multifocal bone LCH. Remodeling following systemic chemotherapy nearly normalizes bone structure in a patient with significant skull lesions. This patient did not have any curettage or excisional surgery in the skull. (B) Brain MRI in a patient with multifocal bone LCH before and after complete excision with placement of mesh grafts. Complete excision of LCH lesion with margins into healthy bone inhibits potential for remodeling. Following resections and successful chemotherapy, skull defects persist.
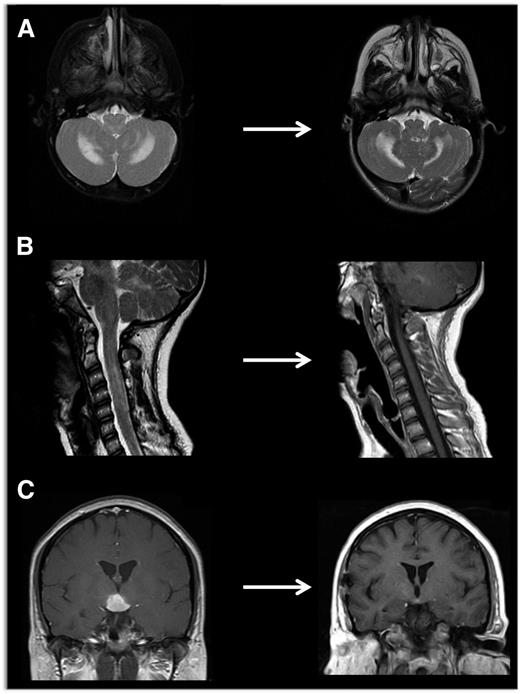
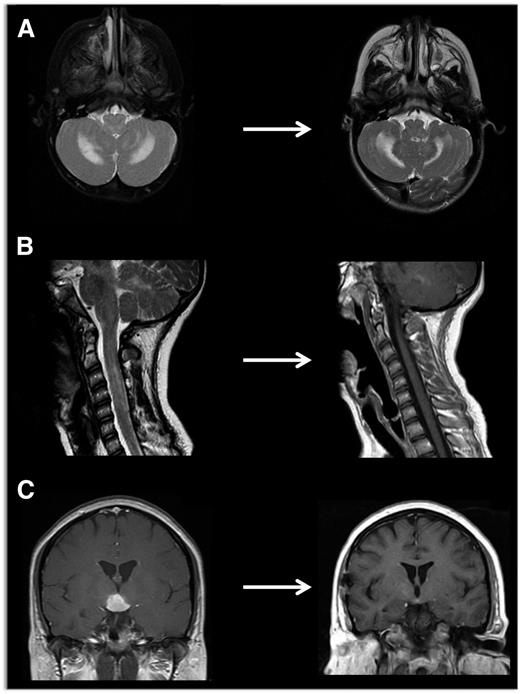
Neuroimaging of LCH lesions. These examples demonstrate typical manifestations of LCH CNS and spinal cord lesions. (A) Brain MRI demonstrates T2-hyperintensity in cerebellum classic for LCH neurodegenerative syndrome. In this case, the patient had radiologic and clinical response to treatment with cytarabine. (B) Spinal MRI demonstrates significant spinal cord lesions. This is a somewhat atypical case of a 13-year-old girl who had marginal response to cytarabine, then clofarabine. BRAF-V600E was detected in cells from the CSF, and the patient ultimately had radiologic and clinical response to vemurafenib. (C) Brain MRI demonstrates a pituitary mass classic for LCH, though differential diagnosis also includes germinoma, lymphoma, and pituitary hypophysitis. In this case, the lesion was biopsy proven to be LCH, and the patient responded to cytarabine therapy.
Neuroimaging of LCH lesions. These examples demonstrate typical manifestations of LCH CNS and spinal cord lesions. (A) Brain MRI demonstrates T2-hyperintensity in cerebellum classic for LCH neurodegenerative syndrome. In this case, the patient had radiologic and clinical response to treatment with cytarabine. (B) Spinal MRI demonstrates significant spinal cord lesions. This is a somewhat atypical case of a 13-year-old girl who had marginal response to cytarabine, then clofarabine. BRAF-V600E was detected in cells from the CSF, and the patient ultimately had radiologic and clinical response to vemurafenib. (C) Brain MRI demonstrates a pituitary mass classic for LCH, though differential diagnosis also includes germinoma, lymphoma, and pituitary hypophysitis. In this case, the lesion was biopsy proven to be LCH, and the patient responded to cytarabine therapy.
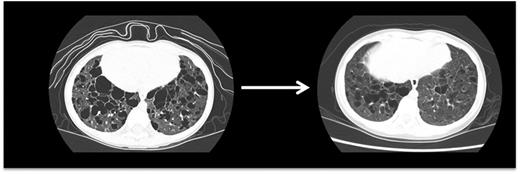
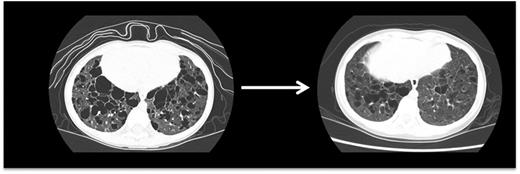
Pulmonary LCH. This high-resolution CT scan demonstrates pulmonary lesions and associated cysts in a 3-year-old girl with high-risk LCH. LCH lesions were cleared from lung parenchyma following cladribine therapy, though some cystic disease was irreversible.
Pulmonary LCH. This high-resolution CT scan demonstrates pulmonary lesions and associated cysts in a 3-year-old girl with high-risk LCH. LCH lesions were cleared from lung parenchyma following cladribine therapy, though some cystic disease was irreversible.
Therapeutic strategies
Consideration of specific presentations
Skin-limited LCH
Cases 1 and 2 illustrate the clinical challenges of infants with skin lesions, who are sometimes assumed to have self-resolving skin-limited LCH. This diagnosis can only be made in retrospect after full evaluation for other sites of disease and then only after the lesions actually resolve30,31 ; in an institutional series, 40% of patients referred for skin-limited LCH had multisystem disease upon further evaluation.26 Infants with true skin-limited LCH have only a remote chance of developing LCH in another organ system, and these rare cases likely represent incompletely evaluated patients at presentation.26 Systemic therapy is appropriate for patients who either are symptomatic with pain, secondary infection, or other complications from persistent lesions. Data for effective therapy for skin-limited disease are limited to case series. Therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; systemic methotrexate, 6-mercaptopurine, vinblastine/vincristine, thalidomide, cladribine, and/or cytarabine.26 We initially treat patients with symptomatic lesions with oral methotrexate (20 mg/m2 weekly) and 6-mercaptopurine (50 mg/m2 per day), then adjust as needed for myelosuppression. Patients with deep ulcerative LCH lesions who do not respond to oral therapy may require systemic chemotherapy.
Single bone lesions
Isolated bone lesions can be effectively treated with limited curettage and/or corticosteroid injection.32 Large pelvic lesions or vertebral lesions not amenable to curettage may be treated with systemic therapy. Radiation therapy may be effective in older children with single vertebral lesions that have not caused complete collapse of the vertebra or with a lesion in the greater trochanter of the femur at risk for pathologic fracture. Although published doses used for treatment of LCH range from 2.5 to a very high 45 Gy (median, 10 Gy),33 we recommend limiting the total dosage to 7.5 Gy in children. Adult series have reported effective outcomes with doses from 10 to 20 Gy.34 The response to radiation therapy is generally excellent, with 90% achieving control of disease. Although there are few reported cases of secondary malignancy following radiotherapy for LCH, the theoretical risk should be considered.35-37
Multiple bone lesions or bone plus another nonrisk site
LCH is rarely fatal in patients with multifocal bone disease, but over half require >1 course of treatment.29,39 The current standard of care as determined by results from the LCH-III trial is to treat these patients with vinblastine/prednisone for 1 year. The frequency of reactivation was significantly decreased by 1 year vs 6 months of therapy,29 suggesting that further prolongation of this mild treatment may further reduce reactivations. This is being tested in the LCH-IV trial.
High-Risk LCH
When an infant presents with hepatomegaly, splenomegaly, or marked cytopenias, the diagnosis of high-risk LCH is straightforward. However, in some cases, risk-organ involvement may be subtle. Hepatic LCH is usually associated with elevated liver enzymes, hypoalbuminemia, or hypoproteinemia. Imaging with ultrasound or MRI may show hypodense areas along the biliary tracts.40 Biopsy of the liver rarely shows CD1a+ cells, but more often lymphocytes and monocytes which infiltrate the portal triads.13 Rarely, mass lesions with CD1a+ cells may also arise in the liver. PET scans are helpful in identifying involvement of the spleen and liver.
As illustrated in case 2, current histologic approaches may be insufficiently sensitive to detect bone marrow involvement.16,41 Based on qPCR of bone marrow aspirate of patients with BRAF-V600E LCH lesions, bone marrow infiltration by BRAF-V600E+ cells is <1% in most cases, and half of the cases reported as histologically normal had detectable cells with BRAF-V600E.16 The relative insensitivity of histological analysis is likely due to variable differentiation and low-level infiltration of LCH precursor cells.
The current standard of care for patients with high-risk LCH is 1 year of therapy with vinblastine/prednisone/mercaptopurine, based on the LCH-III study. Addition of methotrexate to vinblastine/prednisone/mercaptopurine did not impact response or relapse-free survival of high-risk patients. However, increasing treatment duration from 6 months in LCH-II to 12 months in LCH-III was beneficial.42,43 We recommend close follow-up for signs of treatment failure or reactivation for these patients.
In a retrospective institutional series, we found cytarabine monotherapy may be a potentially effective frontline approach,44 though determining relative safety and efficacy compared with vinblastine/prednisone would require a randomized trial.
CNS-risk lesions
In children, bone lesions in the mastoid, sphenoid, orbit, clivus, or temporal bone represent central nervous system (CNS)–risk lesions, indicating increased risk of developing DI and/or neurodegenerative CNS LCH (ND LCH).45 Patients with these “risk” lesions treated with surgery alone or single-agent therapy had a 40% incidence of DI compared with a 20% chance of developing DI when treated for 6 months with vinblastine/prednisone.46 The LCH-III study, in which a year of vinblastine/prednisone was used, showed a further reduction of DI incidence to 12%.29 If patients have refractory or recurrent disease with vinblastine/prednisone, subsequent treatment with cytarabine or clofarabine may be effective.47,48
Special situations
CNS lesions
DI is the most frequent initial sign of LCH in the CNS.49 In children with isolated DI and a thickened pituitary stalk, the most likely diagnoses are LCH, germ cell tumor, or lymphoma.50 LCH is frequently a diagnosis of exclusion with normal germ cell markers and normal CSF cytology. Due to risks of pituitary biopsy, in such patients it is reasonable to initiate LCH therapy empirically and monitor for early response by MRI. Development of DI during or after treatment of another site is considered reactivation and we treat with LCH-directed therapy for an additional year with cytarabine. As illustrated in case 4, patients with long-standing active CNS LCH lesions are at risk for progressive pituitary damage as well as development of ND LCH. In adults with isolated DI, differential diagnosis may also include ECD. Absent other sites of disease in a patient in whom biopsy is not possible, we would evaluate for BRAF-V600E in peripheral blood or cerebrospinal fluid (CSF) to support the diagnosis and identify potential for targeted therapy. Based on experiences with pediatric cases of CNS LCH and juvenile xanthogranuloma (which is histologically similar to ECD), clofarabine may be a reasonable empiric therapy for both LCH and ECD in adults with isolated pituitary lesions.48
Mass lesions of the brain may respond to vinblastine/prednisone, cladribine, cytarabine, and clofarabine.47,48,51,52 Although there has been no trial to suggest which of these regimens might be the best, cytarabine has been effective in a majority of patients and has possible benefit against ND LCH.47 Vinblastine/prednisone is an alternative frontline treatment of pituitary/hypothalamic lesions based on established protocols for multisytem LCH.
Neurodegenerative CNS LCH
Neurodegenerative CNS LCH is a syndrome of uncertain etiology characterized by relentless progression of central neurodegeneration that may occur even 10 years after presumed resolution of LCH lesions.53 Patients may develop clinical symptoms of dysarthria, ataxia, dysmetria, and behavior changes. MRI demonstrates hyperintensity of dentate nucleus and white matter of cerebellum on FLAIR and T2-weighted images, or hyperintense lesions of basal ganglia on T1-weighted images. There is also progressive atrophy of the cerebellum (Figure 6). These diagnostic radiologic findings may precede clinical symptoms by several years.46 A challenge in patients with only radiologic evidence of neurodegeneration is deciding when to treat. We perform regular neurologic exams with the Ataxia Rating Scale on patients with CNS-risk lesions and with LCH ND to document changes in tremor, kinetic functions, speech, and visual abilities.54 A score increased by 5 points indicating clinical deterioration and/or progressive changes on MRI merits consideration of systemic therapy. Similarly, we perform annual brain MRI on patients at risk to screen for development of LCH ND. Early intervention in patients with evolving symptoms is critical. IV γ-globulin and retinoic acid have been reported to stabilize progression of LCH ND.55,56 In an institutional series, vincristine/cytarabine was associated with improvement in clinical symptoms and MRI images in 6 of 8 patients.47 We therefore favor this strategy for patients with LCH ND. Some patients fail to respond to these therapies and have an inexorable decline in neurologic function over the course of years. As the etiology of ND LCH remains uncertain, it is not known whether MAPK inhibition could impact development or progression of ND LCH.
LCH in adults
Data for treatment of adults with LCH are limited to case reports and case series with no prospective clinical trials to inform therapeutic strategies. In general, we follow the same diagnostic and therapeutic approaches in adults as in children, with some specific considerations outlined in the following sections.
Multifocal LCH
We found that vinblastine/prednisone according to standard pediatric protocol had near universal toxicity and suboptimal efficacy in adults.57 We therefore initially treat adults with either multifocal bone disease, bone and other site, or lesions in risk organs with cytarabine (100 mg/m2 per dose × 5 days per month × 12 months). Patients with CNS lesions, CNS-risk lesions, or progressive neurodegeneration (as in case 4) are treated with a higher dose (150 mg/m2 per dose).
Oncologists less familiar with LCH sometimes advocate for extreme surgical procedures. As with isolated pediatric bone lesions, “clean” surgical margins are not required, and only a diagnostic biopsy should be performed in patients with multifocal disease in whom systemic therapy is indicated.
LCH in adults can arise as a component of mixed histiocytic disease with ECD, with mixed phenotype in the same lesion or as a distinct phenotype of separate lesions.9,58 ECD generally has poorer outcome than LCH and may benefit from alternative initial therapy.59 PET scan, MRI of chest (cardiac involvement), abdomen (kidney and aortic involvement), and legs (tibial involvement), and biopsy of multiple accessible lesions in adults with LCH may be informative to identify patients with simultaneous ECD.
Pulmonary LCH
Case 3 illustrates the classic presentation of isolated pulmonary LCH in adults who smoke, which may resolve with smoking cessation alone. If lesions persist or progress, systemic therapy is indicated, initially with corticosteroids. Patients with persistent disease despite smoking cessation and a steroid trial may require chemotherapy. Although there are more case series to support use of cladribine for refractory pulmonary LCH,60-62 we favor cytarabine due to efficacy in adults with multisystem LCH and the association of cladribine with bone marrow aplasia.57 In extreme cases, lung transplant may be necessary due to the extent of parenchymal injury.
Pain and fatigue syndrome in adults with LCH
Many adult LCH patients are afflicted with chronic pain and fatigue of uncertain etiology. Pain may localize to specific sites despite lack of identifiable pathology. Treatment with duloxetine, gabapentin, narcotics, or bisphosphonates may ameliorate symptoms. Fatigue in adult LCH patients may also be associated with panhypopituitarism, hypoadrenalism, or hypothyroidism. Complete evaluation for these endocrinopathies is critical.63
Salvage therapy
Despite almost universal survival of patients with “low-risk” disease, patients with persistent or recurrent LCH suffer from significant morbidities including pain, pituitary dysfunction, growth retardation, hearing loss, sclerosing cholangitis, and progressive neurodegenerative disease.64 Over 50% of patients with LCH will be refractory to initial therapy or develop recurrent disease, with the majority of reactivations occurring in the first 2 years.29,39,43 Long-term follow-up is therefore critical. Repeat imaging depends on the site of disease and response to therapy (Table 1).
We consider the standard of care for initial therapy to be vinblastine/prednisone according to LCH-III in patients requiring systemic treatment. Many patients who fail to achieve a durable response are ultimately cured with salvage therapies that include agents used in treatment of acute myeloid leukemia, including cytarabine, cladribine, and clofarabine.57,65-67 Lower-dose cladribine (5 mg/m2 per day × 5 days per month × 6 months) was effective in achieving a response in patients who were refractory to frontline therapy with response rates of 22% in patients with risk-organ involvement and 62% in patients without risk-organ involvement, but only 4% were cured by week 24.65 Intermediate-dose cytarabine (100-170 mg/m2 per day) was associated with 41% progression-free survival in an institutional series.44 By comparison, a salvage strategy based on a much higher dose combination of nucleoside analogs cytarabine (1 g/m2 per day) and cladribine (9 g/m2 per day × 5 days per cycle) was universally effective in achieving cure in surviving patients, but was associated with extremely high treatment-related toxicity.68
The recent identification of immature myeloid precursors at the origin of LCH pathogenesis may explain the relative efficacy of the purine analogs in LCH.16 Clofarabine is a second-generation nucleoside analog with activity in refractory acute myeloid leukemia.69 Case series have reported clofarabine monotherapy as a successful salvage therapy in LCH patients who failed to achieve a durable response to cladribine or cytarabine.48,66,70 One-year progression-free survival was 76% for 11 LCH patients who had failed a median of 3 previous chemotherapy strategies, and most patients (64%) had complete responses after 6 months of therapy.48 Data from phase 1 studies define the maximum tolerated dose in children as 52 mg/m2 per day × 5 days.71 The majority of patients in the LCH series received 25 mg/m2 per day (5 days per cycle) for 6 cycles as outpatients, with minimal toxicity beyond predicted cytopenias. These data suggest that nucleoside analogs are effective in treating LCH, with dose-dependent effects on therapeutic efficacy as well as toxicity. Patients with persistent high-risk LCH despite adequate attempts at salvage therapy may be cured with stem cell transplant, with improved outcomes with reduced-intensity conditioning.72,73
Late effects
Morbidity associated with disease as well as therapy remains a major challenge in LCH.64 Patients lagging in growth or with other clinical signs concerning for pituitary dysfunction should be referred for evaluation of possible polyendocrinopathies. Patients with vertebral lesions are at risk for chronic instability of the spine with pain and limitation of motion. When LCH causes extensive damage to the bile ducts, progressive sclerosing cholangitis may develop, most often resulting in liver failure with the need for liver transplantation.74 The severity of late effects is associated with extent and duration of active disease.39,46,64 We therefore treat all patients with active LCH with curative intent, with the possible exception of otherwise asymptomatic infants with skin lesions. All patients should be monitored closely for neuropathy, learning difficulties, and growth and development abnormalities. Yearly brain MRIs and neuropsychological evaluations can identify development or progression of LCH ND in patients with history of DI or CNS-risk skull lesions.
The future of LCH
Clinical trials
The standard of care presented in this manuscript and improvements in outcomes are based largely on cooperative randomized clinical trials organized by the Histiocyte Society. Important concepts that have emerged from Histiocyte Society trials include benefit of dose intensification,43 as well as decreased reactivation with therapy prolongation.29
The most recent Histiocyte Society trial, LCH-IV, is now open for pediatric patients. The broad goals are to determine optimal therapy for all patients, as stratified: high-risk multisystem, low-risk multisystem, low-risk single system, and LCH with special site involvement, particularly focusing on the difficult problem of diffuse CNS disease. We encourage enrollment of newly diagnosed patients with LCH in this study. The randomized studies in the LCH-IV protocol seek to optimize the outcomes of first line treatments by testing prolonging (12 vs 24 month) and intensifying (±6-mercaptopurine) treatment of high risk patients, and by comparing 6- vs 12-month treatment for single system disease. The protocol also embodies a randomized study of new combinations as second-line treatment of individuals with low-risk disease either not initially responding or reactivating. Finally, it will explore whether either IVIg or cytarabine impact the development or progression of neurodegenerative LCH.
Targeted therapy
Targeted inhibition of MAPK activation is an obvious therapeutic strategy for LCH (Figure 2).15,20,21 An institution reported a series of adults with ECD and/or LCH treated with vemurafenib with promising responses.75 Larger series with longer follow-up will be required to determine the potential for cure and toxicity. In melanoma trials, vemurafenib is associated with a complex toxicity profile, including secondary squamous cell carcinoma in over 30% of patients, and case reports of more serious adenomas.76-78 Because LCH is almost universally cured with chemotherapy, the question of acceptable risk and relative efficacy of agents that have not undergone phase 1 testing in children remains unanswered. We favor development of clinical trials to test novel agents in patients who have persistent or progressive severe disease after salvage chemotherapy. As we gain more experience with these agents in children, they may move to earlier use alongside or instead of chemotherapy.
Personalized therapy
New insights into LCH pathogenesis suggest that patients have individual routes to LCH that depend on the MAPK mutation, the state of differentiation in which the mutation arises, genetic background, and other as-yet-unidentified factors. As patients are treated on prospective clinical trials, correlative biology studies will inform the clinical utility of risk stratification based on mutation, differentiation of cells harboring the mutation, persistence of cells in circulation, plasma/CSF protein profiles, or other biomarkers of disease burden and response to therapy, including assays for specific mutations in peripheral blood (or CSF or urine).16,79
Conclusions
Concepts of LCH, including hematopoietic origin and optimal therapeutic approaches, continue to evolve. Emerging data support therapeutic strategies aimed at cure by eliminating the pathologic clonal cells as opposed to “watch and wait” or episodic approaches sometimes used for autoimmune disease. Increasing patient enrollment in clinical trials to test novel strategies will further catalyze improvements. With reconsideration of LCH as a myeloproliferative neoplasia, we hope that cooperative clinical trial groups and funding agencies will embrace LCH and support research to improve outcomes for children and adults with LCH.
Acknowledgments
The authors acknowledge the long-term support and extraordinary efforts of the Histiocytosis Association and the Histiocyte Society in promoting translational and clinical research in LCH through research grants and clinical trials. The authors also thank Dr John Hicks for sharing histology images.
The Texas Children’s Cancer and Hematology Centers (TXCH) Histiocytosis Program is supported by the HistioCure Foundation. Grant support includes National Institutes of Health (NIH) National Cancer Institute (NCI) R01 (CA154489) (C.E.A., K.L.M.) and NIH NCI Specialized Programs of Research Excellence (SPORE) in Lymphoma (P50CA126752) (C.E.A.). This manuscript is a collaborative project with support from St. Baldrick’s Foundation, which sponsors the North American Consortium for Histiocytosis Research (NACHO; C.E.A., K.L.M., S.L.).
Authorship
Contribution: C.E.A., S.L., and K.L.M. conceived, organized, and wrote this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephan Ladisch, Center for Cancer and Immunology Research, Children’s Research Institute, Children’s National Medical Center, 111 Michigan Ave NW, Washington, DC 20014; e-mail: sladisch@childrensnational.org; and Kenneth L. McClain, Texas Children’s Cancer Center, Baylor College of Medicine, 6701 Fannin St, Suite 1510, Houston, TX 77030; e-mail: klmcclai@txch.org.
References
Author notes
C.E.A., S.L., and K.L.M. contributed equally.