Abstract
Systemic anaplastic large cell lymphoma (ALCL) is an aggressive CD30+ non-Hodgkin lymphoma. Anaplastic lymphoma kinase–positive (ALK+) ALCL is associated with the NPM-ALK t(2;5) translocation, which is highly correlated with the identification of the ALK protein by immunohistochemistry. ALK+ ALCL typically occurs in younger patients and has a more favorable prognosis with 5-year survival rates of 70% to 90% in comparison with 40% to 60% for ALK-negative (ALK−) ALCL. Studies support young age as a strong component of the favorable prognosis of ALK+ ALCL. Until recently, no recurrent translocations were identified in ALK− ALCL. However, emerging data now highlight that ALK− ALCL is genetically and clinically heterogeneous with a subset having either a DUSP22 translocation and a survival rate similar to ALK+ ALCL or a less common P63 translocation, the latter associated with an aggressive course. Anthracycline-based regimens such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) remain the standard first-line treatment choice for systemic ALCL, but in many patients with ALK− ALCL, it is ineffective, and thus it is often followed by consolidative autologous stem cell transplantation. However, selection of appropriate patients for intensified therapy remains challenging, particularly in light of genetic and clinical heterogeneity in addition to the emergence of new, effective therapies. The antibody drug conjugate brentuximab vedotin is associated with a high response rate (86%) and durable remissions in relapsed/refractory ALCL and is under investigation in the first-line setting. In the future, combining clinical and genetic biomarkers may aid in risk stratification and help guide initial patient management.
Anaplastic large cell lymphoma: historical perspective
In 1985, Stein and colleagues1 identified a subset of non-Hodgkin lymphomas (NHLs), termed “Ki-1 lymphomas” characterized by large CD30+ (Ki-1) anaplastic cells, which have a tendency to grow cohesively and a predilection to invade lymph node sinuses. Although most cases were T-cell or null-cell lineage, 15% had a B-cell phenotype. In the Revised European American Lymphoma classification, the name of this specific subset was updated to anaplastic large cell lymphoma (ALCL) and confined to cases that were T-cell or null-cell type.2 Subsequently, several groups identified the presence of a translocation involving the anaplastic lymphoma kinase (ALK) gene on chromosome 2p23 and the nucleophosmin (NPM) gene on chromosome 5q35 that formed a novel chimeric fusion protein, NPM-ALK.3 Subsequent studies confirmed the favorable prognosis of ALK-positive (ALK+) ALCL (Table 1). In the World Health Organization (WHO) classification, primary cutaneous ALCL (PCALCL) was separated from systemic ALCL because of its indolent behavior and favorable prognosis.4 In addition, the provisional category of “Hodgkin-like ALCL” was removed, with emerging molecular genetics and immunophenotyping information now being available to classify borderline cases as either Hodgkin lymphoma (HL) or ALCL. In 2008, systemic ALCL was officially separated into ALK+ ALCL which was recognized as a distinct entity, and ALK-negative (ALK−) ALCL, which was still considered a provisional entity because of a lack of defining characteristics.5 However, recent genetic advances have secured ALK− ALCL as a distinct entity in the upcoming revision of the WHO classification (E. Jaffe, WHO classification author, personal communication January 2015). This review will focus on advances in understanding the biology and pathogenesis of adult systemic ALCL and will provide a critical review of studies evaluating prognosis and management.
Epidemiology and clinical features of ALK+ and ALK− ALCL
ALCL comprises approximately 3% of all adult NHLs6 and 10% to 20% of childhood lymphomas.7 The overall frequency of ALK+ ALCL depends on the population studied because it is more commonly seen in children and young adults with a median age of 30 years, whereas ALK− ALCL occurs in older adults (median age, 55 years).8-10 For both types, the majority of patients are male and present with advanced stage III to IV disease, often with B symptoms. Extranodal sites frequently occur and include skin, soft tissue, bone, lung, and liver, as well as bone marrow.8,9,11 Central nervous system involvement can occur at diagnosis or at relapse.12,13 Of note, systemic ALCL should be distinguished from PCALCL, an indolent entity with disease-specific survival rates of 85% to 95%.14 Thus, all patients with PCALCL should have standard staging procedures to rule out systemic involvement.
Pathology
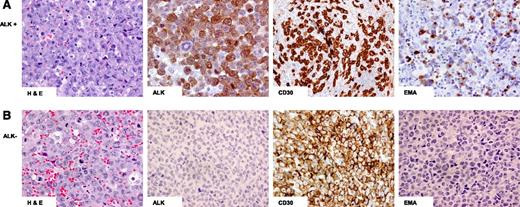
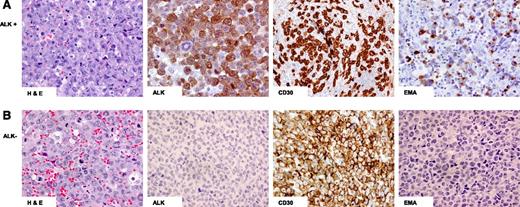
Morphologically, ALCL demonstrates a variable proportion of hallmark cells characterized by eccentrically placed horseshoe- or kidney-shaped nuclei with an intermediate nuclear:cytoplasmic ratio and eosinophilic perinuclear clearing15 (Figure 1). In most cases of ALK+ and ALK− ALCL, the nodal or tissue architecture is effaced by solid cohesive sheets of neoplastic cells, although a sinusoidal pattern of infiltration is frequently seen in lymph nodes.
Hematoxylin and eosin (H&E) and immunohistochemical staining of ALCL. (A) ALK+: Hallmark cells demonstrated by H&E staining and tumor cells positive for ALK, CD30, and epithelial membrane antigen (EMA); (B) ALK−: Hallmark cells demonstrated by H&E staining and tumor cells positive for CD30 but negative for ALK and EMA staining.
Hematoxylin and eosin (H&E) and immunohistochemical staining of ALCL. (A) ALK+: Hallmark cells demonstrated by H&E staining and tumor cells positive for ALK, CD30, and epithelial membrane antigen (EMA); (B) ALK−: Hallmark cells demonstrated by H&E staining and tumor cells positive for CD30 but negative for ALK and EMA staining.
There are 5 morphologic patterns of ALK+ ALCL: common, lymphohistiocytic, small cell, Hodgkin-like, and composite.5 Most patients demonstrate the common type with sheets of large lymphoid cells featuring hallmark cells.10 The lymphohistiocytic pattern (10%) consists of reactive histiocytes that may mask the anaplastic tumor cells. The small cell pattern (5% to 10%) consists of small- to medium-size cells that can be misdiagnosed as peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS). Both the lymphohistiocytic and small cell variants are more common in children and can often be misdiagnosed as benign infiltrates. The Hodgkin-like pattern (3%) may resemble nodular-sclerosis classical HL with tumor nodules surrounded by fibrous bands. The tumor cells in ALK− ALCL demonstrate similar heterogeneity; however, a small cell pattern is not recognized.
ALCL was originally distinguished by the discovery of CD30 expression in lymphomas with an anaplastic morphology1 (Figure 1). This was followed by the discovery of a balanced t(2;5)(p23;q25) chromosomal translocation in a subset of patients and involved the ALK gene on chromosome 2p23 and the NPM gene on chromosome 5q35 forming the novel chimeric protein NPM-ALK. The t(2;5) translocation occurs in approximately 75% to 85% of all ALK+ ALCL patients, and in the remaining patients, there is a variant rearrangement that involves 2p23 and a multitude of partner genes.5 These variant translocation partners can be recognized because of different ALK protein staining patterns. Subsequent studies have shown that the NPM-ALK chimeric protein has constitutive activation of the ALK tyrosine kinase.16
The detection of ALK protein correlates nearly 100% with the presence of a chromosomal rearrangement involving ALK; thus immunohistochemistry (IHC) has largely replaced molecular testing in ALCL. It is recommended that monoclonal antibodies (mouse or rabbit) be used instead of polyclonal antibodies, which may lead to false positives.5 Because ALK+ and ALK− ALCL are morphologically indistinguishable, ALK IHC is critical in all cases. ALK expression is absent from all postnatal normal human tissues except for rare cells in the brain.17,18 ALK staining is cytoplasmic and nuclear in cases of the classic t(2;5)/NPM-ALK translocation but may be membranous or diffuse/granular cytoplasmic in cases with a variant translocation.19,20 Although IHC for ALK is highly sensitive, it is not specific for ALK+ ALCL. Rare cases of ALK+ lung cancers and other solid tumors have been described,21 in addition to ALK+ diffuse large B-cell lymphoma (DLBCL),22 the latter characterized in most cases by t(2;17)(p23;q23), which encodes for a clathrin-ALK fusion protein.23 These cases are easily distinguished from ALCL on the basis of morphologic and immunophenotypic criteria. Importantly, ALK+ DLBCL does not express CD30.
The aberrant loss of pan T-cell antigens is characteristic of ALCL, and 20% have a null immunophenotype,15 but nearly all have a clonal T-cell receptor (TCR) gene rearrangement.24 ALK+ and ALK− ALCL can differ immunophenotypically (Table 2).25 CD3 is negative in most ALK+ ALCL, whereas a greater proportion of ALK− ALCL tumors are CD3 positive as well as CD2 positive.8,26 The majority of ALK+ cases are positive for epithelial membrane antigen (EMA), but it is less common in ALK− ALCL8,27 (Table 2 and Figure 1). Most cases express cytotoxic markers but are CD8 negative.5
Differential diagnosis of ALK− ALCL
ALK− ALCL vs CD30+ PTCL-NOS
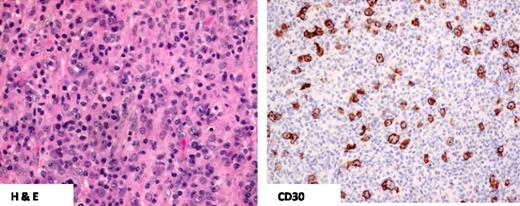
The pathologic distinction between ALK− ALCL and CD30+ PTCL-NOS can be difficult (Figures 1 and 2). In general, PTCL-NOS is more likely to be CD2+ and CD3− but epithelial membrane antigen negative, and it usually lacks cytotoxic proteins8,25 (Table 2). The distinction is more challenging in PTCL-NOS cases with high expression of CD30. Recently, a 3-gene model (TNFRSF8, BATF3, and TMOD1) was validated by using reverse transcription polymerase chain reaction in formalin-fixed paraffin-embedded tissue that was able to distinguish ALK− ALCL from PTCL-NOS, including CD30+ PTCL-NOS, but it is not yet routinely applied in clinical practice.27 Interestingly, one study suggested some biologic overlap between CD30+ PTCL-NOS and ALK− ALCL when both entities had low expression of TCR signaling and T-cell differentiation proteins.28
CD30+ PTCL-NOS. H&E staining demonstrates predominantly small- to medium-size lymphocytes with pleomorphism and the absence of hallmark cells. Only scattered tumor cells demonstrate CD30 positivity compared with ALCL.
CD30+ PTCL-NOS. H&E staining demonstrates predominantly small- to medium-size lymphocytes with pleomorphism and the absence of hallmark cells. Only scattered tumor cells demonstrate CD30 positivity compared with ALCL.
ALK− ALCL vs HL
HL tumors rich in Hodgkin-Reed-Sternberg cells with lymphocyte depletion and a less prominent mixed inflammatory infiltrate may be misdiagnosed as ALK− ALCL.29 CD30 and PAX-5 are useful in this instance because HL is usually weakly positive for PAX-5, whereas ALK− ALCL is negative and CD30 is typically weaker and more heterogeneous in HL.
Molecular genetics and gene expression profiling in ALK+ and ALK− ALCL
Comparative genomic hybridization demonstrates that ALK+ and ALK− ALCL harbor different genetic aberrations (Table 3). Overall, secondary genetic imbalances occur in 58% of ALK+ and 65% of ALK− ALCL. Gains of 7, 17p, and 17q and losses of chromosome 4, 11q, and 13q have been observed in ALK+ ALCL. Conversely, ALK− ALCL harbors gains of 1q and 6p2130 (Table 3).
Gene expression profiling studies support a shared origin of ALCL, but distinct signatures can also be seen that have been used to aid molecular classification.31-34 One study demonstrated that ALK+ and ALK− ALCLs share a cluster of transcripts, indicating that ALK-independent genes may be part of a common signature that distinguishes them from other PTCLs.32 Recently, it has also been shown that both subtypes of ALCL are dependent on IRF and MYC signaling.35 Iqbal and colleagues,33 developed an ALCL molecular signature that included genes previously identified as having high expression in ALCL, including CD30 (TNFTFSF8), BATF3, and TMOD. In addition, there was low expression of genes associated with TCR signaling, as previously described.33,36 A gene signature also distinguished ALK+ ALCL from ALK− ALCL and had high concordance with pathologic diagnoses. ALK+ ALCL was enriched for HIF1-α target genes as well as interleukin-10 and H-ras/K-ras–induced genes,33 whereas ALK− ALCL was enriched for PI3K pathway–regulated genes and all cases expressed TNFRSF8, GATA3, and TMOD1 in keeping with the described 3-gene model.27 In comparison with PTCL-NOS, ALK− ALCL was enriched for MYC and IRF4 target gene signatures as well proliferation and MTOR gene signatures.33 A separate genome-wide profiling study also found that PRDM1/BLIMP1 is commonly inactivated in ALK− ALCL and may be associated with a more aggressive course.37
Next-generation sequencing recently identified 2 recurrent rearrangements in ALK− ALCL38,39 (Table 3). One involves the P53 homolog P63 on 3q28, and the other involves the DUSP22-IRF4 locus on 6p25.3 (DUSP22 rearrangement). The presence of a DUSP22 rearrangement was associated with reduced protein expression.38,39 An analysis of 73 patients with ALK− ALCL identified DUSP22 and P63 rearrangements in 30% and 8% of ALK− ALCL patients, respectively, but they were absent in ALK+ ALCL.40 These rearrangements were mutually exclusive and appear to have important prognostic relevance (see “Prognostic factors in ALCL”).
Collectively, these key advances defining the unique features of ALK− ALCL have now secured it as a distinct entity in the upcoming revised WHO classification of lymphomas (E. Jaffe, WHO classification author, personal communication, January 2015).
Prognostic factors in ALCL
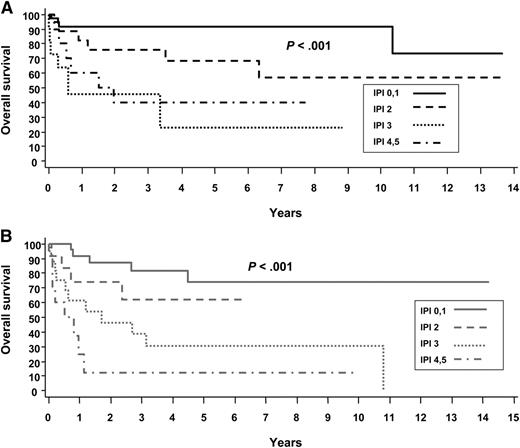
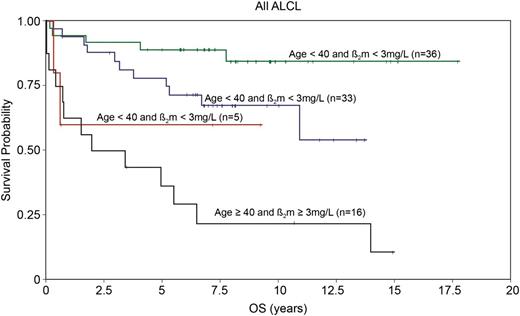
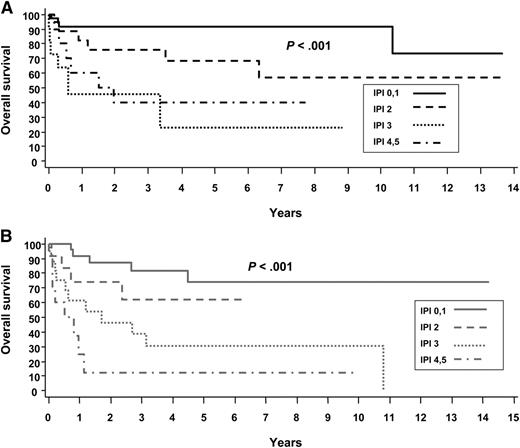
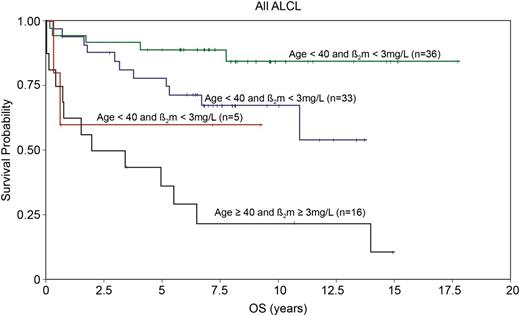
The International Prognostic Index (IPI) is a clinical risk stratification model developed in aggressive lymphomas, primarily DLBCL.41 The IPI is also effective in stratifying many of the PTCL subtypes, including ALCL. In the International Peripheral T-Cell Lymphoma project, the 5-year overall survival (OS) by low (0-1 risk factors), low-intermediate (2 risk factors), high-intermediate (3 risk factors), and high-risk (4-5 risk factors) IPI in ALK+ and ALK− ALCL were 90% vs 74%, 68% vs 62%, 23% vs 31%, and 33% vs 13%, respectively8 (Figure 3). These data highlight that in addition to ALK status, clinical factors are important in estimating prognosis. Numerous other studies have also reported the usefulness of the IPI in risk stratifying patients with ALCL.11,26,42-45 It is notable that overall, these studies demonstrate that patients with ALK+ ALCL with 3 or more IPI risk factors have a 5-year progression-free survival (PFS) rate of 20% to 30%, similar to that in other PTCLs. Conversely, patients with low-risk ALK− ALCL can have a favorable prognosis. This is underscored in a study by GELA (Groupe d’Etude des Lymphomes de l’Adulte), which demonstrated that age is a prominent factor driving the prognostic difference between ALK+ and ALK− ALCL. In a survival comparison limited to patients younger than age 40 years, outcomes were similar in ALK+ and ALK− ALCL,11 which was also observed in another study.8 In addition to age <40 years, the GELA study also established that low β2-microglobulin (<3 mg/dL) was a favorable prognostic factor.11 Patients with neither factor had an 8-year OS of 84%, and those with both factors had an OS of only 22% (P < .001)11 (Figure 4). The model was particularly effective in defining a very favorable low-risk group of patients with ALK− ALCL who had an 8-year OS of 100% (8-year PFS, ∼85%).11
Overall survival of ALCL by the International Prognostic Index (IPI). (A) ALK+ and (B) ALK−. Reprinted from Savage et al with permission.
Overall survival of ALCL by the International Prognostic Index (IPI). (A) ALK+ and (B) ALK−. Reprinted from Savage et al with permission.
Overall survival in ALCL patients according to age (<40 or ≥40) and B2-microglobulin (normal or abnormal). Adapted and reprinted from Sibon et al11 with permission.
Overall survival in ALCL patients according to age (<40 or ≥40) and B2-microglobulin (normal or abnormal). Adapted and reprinted from Sibon et al11 with permission.
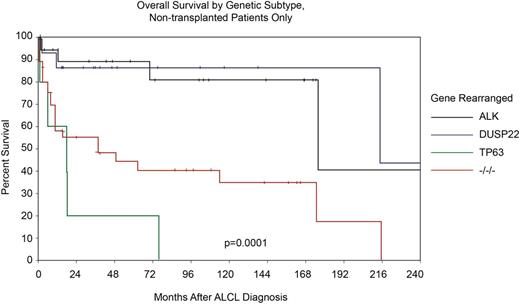
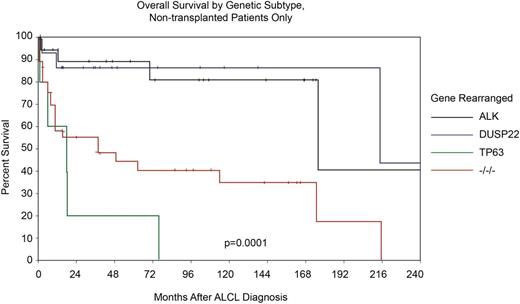
As described, two new recurrent chromosomal rearrangements involving DUSP22 and P63 were recently identified in ALK− ALCL, highlighting additional genetic heterogeneity that also appears to be clinically relevant40 (Table 3). For the majority of cases of ALK− ALCL that lack either rearrangement (also known as triple negative), the 5-year OS rate was 42%, which is similar to estimates reported in many series lacking genetic information (Table 1 and Figure 5). However, cases of ALK− ALCL that harbor a DUSP22 rearrangement had a 5-year OS rate that was indistinguishable from that of a control group of ALK+ ALCL patients (5-year OS, 90% for DUSP22+ ALK− ALCL vs 85% for ALK+ ALCL), which all lacked DUSP22 translocations.40 Conversely, patients with rearranged TP63 had extremely poor prognoses with a 5-year OS of only 17%. Adjusting for the IPI in multivariate analysis and using ALK+ as the reference, both TP63 and triple-negative patients had an inferior prognosis, but patients with DUSP22 ALK− ALCL had a favorable outcome, as did those patients who did not undergo transplantation (Figure 5).
Overall survival stratified by ALK, DUSP22, TP63 translocations and triple negative status in patients with ALCL who did not undergo transplant. Adapted and reprinted from Parilla Castellar with permission.
Overall survival stratified by ALK, DUSP22, TP63 translocations and triple negative status in patients with ALCL who did not undergo transplant. Adapted and reprinted from Parilla Castellar with permission.
The genetic heterogeneity in ALK− ALCL may also explain discordant study results comparing the prognosis of ALK− ALCL and CD30+ PTCL-NOS. Some studies have reported that CD30+ PTCL-NOS is associated with an inferior outcome compared with ALK− ALCL8,34 ; however, others have demonstrated a nonsignificant improvement in outcome.28 The discrepancy may reflect the presence of unadjusted clinical or genetic factors.
Although further validation of the prognostic importance of DUSP22 and TP63 rearrangements is warranted, these data support the existence of important genetic heterogeneity within ALK− ALCL, which has an impact on prognosis and is relevant in comparing outcomes between studies and evaluating the impact of treatment regimens, including the role of transplantation.
Management of systemic ALCL
Because of disease rarity, there are currently no randomized controlled trials (RCTs) to guide treatment decisions in ALCL, and as a result, the optimal therapy remains unknown. The majority of evidence describing outcomes for adult patients with systemic ALCL and the impact of various treatment regimens comes from retrospective studies or subgroup analyses of completed prospective studies in aggressive lymphomas or PTCLs.
Primary therapy for systemic ALK+ and ALK− ALCL
Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) is the standard chemotherapy for aggressive lymphomas, including ALCL.46 For ALK+ ALCL, outcomes with CHOP or CHOP-like regimens are generally favorable (Table 1), with the exception being those patients with multiple IPI risk factors. Considering patients of all ages, the outcome in ALK− ALCL is consistently worse when using CHOP-like regimens than in ALK+ ALCL, but it is also much more variable across studies, with 5-year OS rates from 15% to 62% which likely reflect disease and clinical heterogeneity (Table 1). The GELA group reviewed 138 patients with ALCL (64 ALK+, 74 ALK−) prospectively treated across multiple trials from 1997 to 2010, including 3 unpublished trials.11 The most commonly received regimen was doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (ACVBP) followed by sequential consolidation with methotrexate, ifosfamide, etoposide, and cytarabine and, in some cases, high-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT).11 Overall, the outcome of ALK+ ALCL was superior to that of ALK− ALCL (Table 1); however, it was similar in patients <40 years old, with an 8-year OS rate of more than 80% in both groups. Although this study investigated a regimen that is more intensive than CHOP, it supports the finding that young low-risk patients with ALK− ALCL have outcomes similar to patients with ALK+ ALCL.
Several studies have evaluated the impact of more dose-intensive or alternate chemotherapy strategies in PTCLs, but because of disease rarity, they have largely combined all subtypes in outcome analyses. A US multicenter retrospective analysis evaluated the outcome of PTCLs, including 88 patients with ALCL (23 ALK+, 43 ALK−, 22 ALK status unknown) and did not demonstrate improved survival with the dose-intensive hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine (hyperCVAD) regimen, but the impact in ALCL was not analyzed.47 Similarly, the MD Anderson Cancer Center retrospectively evaluated the survival of 135 PTCL patients by type of treatment regimen received, including 40 patients with ALCL (12 ALK+, 19 ALK−, 9 unknown) and found no improvement in outcome using dose-intensive chemotherapy, but again, all subtypes were combined.48 CHOP was compared with dose-intensive etoposide, ifosphamide, and cisplatin (VIP) plus doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) in a phase 3 RCT that included 32 patients with ALCL (22 ALK+, 10 ALK−) and showed similar outcomes between the treatment arms.49
It has been suggested that the addition of etoposide may improve outcome in PTCLs, including ALCL. This is largely based on the German Non-Hodgkin Lymphoma Group (DSHNHL) retrospective analysis of 289 patients with PTCL subtype enrolled onto completed prospective aggressive lymphoma studies, which included 78 ALK+ and 113 ALK− ALCL patients. For select young good-risk patients (age <60 years; normal lactate dehydrogenase), the addition of etoposide improved the 3-year event-free survival (EFS) (70.5% vs 51%; P = .003). The impact was most evident in ALK+ ALCL (3-year EFS, 91% vs 57%), and a similar trend was observed for other nodal PTCLs, which included ALK− ALCL (60.7% vs 48.3%; P = .057).42 However, for all comparisons, the OS was not statistically different, and the analyses were not adjusted for the IPI. Excluding ALK+ ALCL patients, a Swedish registry study showed use of cyclophosphamide, doxorubicin, etoposide, vincristine and prednisone (CHOEP) was associated with an improvement in PFS (P = .008) in multivariate analysis with a trend toward an improved OS (P = .052) in PTCL patients age <60 years.43 However, there was no improvement of PFS or OS if an upper age limit of 70 years was used, and efficacy in ALCL was not reported. The US retrospective study did not find a benefit of etoposide (P = .80), but patient numbers were small.47 Further studies are needed to evaluate the added benefit of etoposide in first-line therapy for PTCLs, including ALCL. Of note, several studies have also evaluated the addition of another agent to CHOP, including targeted therapies and monoclonal antibodies such as alemtuzumab, in an effort to improve outcome in PTCLs. However, a detailed review is outside the scope of this article.
After induction chemotherapy with CHOP, patients with ALK− ALCL often receive a consolidative transplantation in first complete remission (CR1), but it remains challenging to know which patients to select for this intensified approach. The Nordic group completed the largest prospective phase 2 trial (NLG-T-01) in 160 patients with PTCL, which included 31 patients with ALK− ALCL.50 The planned treatment schedule was CHOEP (or CHOP14 for patients age >60 years) for 6 cycles followed by carmustine, etoposide, cytarabine, and melphalan (BEAM)/ASCT in responding patients. The transplantation rate was 70%, and with a median follow-up of almost 4 years, the 5-year PFS was 44% and 5-year OS was 51% for all patients but was superior in ALK− ALCL compared with nonanaplastic subtypes with a 5-year PFS of 61% (P = .04) and 5-year OS of 70% (P = .03). ALK− ALCL remained a favorable prognostic factor in multivariate analysis.50 It would be interesting to determine the frequency of DUSP22 rearrangements in this subgroup. In contrast to these results, the DSHNHL retrospectively evaluated the outcome of 33 patients with T-cell NHL, primarily PTCLs, including 39% with ALK− ALCL who were treated with intensified high-dose CHOP and etoposide (CHOEP) and SCT on phase 2 or 3 trials and reported a disappointing 3-year EFS of only 26%.51 In the US retrospective study, a multivariate analysis was performed that controlled for CR to initial therapy; it failed to demonstrate a benefit of consolidative ASCT, but ALCL patients were not evaluated separately.49
There may still be a role for consolidative ASCT in the primary treatment of ALK− ALCL, but more information is needed to select high-risk patients who may benefit. Ideally, treatment would incorporate clinical and genetic factors, particularly in the landscape of new highly effective therapies (see below). Conversely, ALK+ ALCL patients with a high IPI score have poor outcomes, and alternate strategies should be considered for this group.
Limited-stage ALCL
The majority of patients with ALCL present with advanced-stage disease, but a subset of patients present with limited-stage disease. The largest study evaluated the outcome of 46 patients with early-stage systemic ALCL (stage I, n = 20; stage II, n = 26) and demonstrated favorable outcomes with primarily short-course CHOP-based chemotherapy with planned radiotherapy (RT). In 39 patients with ALK status information available, 54% were ALK− and 46% were ALK+. Overall, the 5-year PFS and OS were 64% and 86%, respectively.52 There was a trend toward improved outcomes in patients with stage I compared with stage II disease (5-year PFS, 78% vs 54%; P = .078; 5-year OS, 95% vs 79.5%; P = .075). In contrast, a more recent study evaluated the outcome of 75 patients with PTCL, including 35 patients with ALCL (40% ALK+, 40% ALK−, 6% unknown) and failed to show an improved outcome with RT in an analysis restricted to those with responding disease. However, the patient numbers were small and specific information for ALCL patients was not provided.53 None of these studies establish the optimal number of cycles of chemotherapy, but following a similar approach for limited-stage DLBCL is appropriate.
Breast implant–associated ALCL
Breast implant–associated ALCL (BIA ALCL) was first described in 1997,54 and after multiple cases were reported, the US Food and Drug Administration (FDA) issued a statement noting the increased risk of ALCL in women who had breast implants.55 A long-term follow-up of 60 published cases of BIA ALK− ALCL was recently reported.56 The tumor was confined to the capsule in 42 patients, whereas there was a tumor mass in 18 patients. Capsulectomy and implant removal were performed in 93% of the patients. Therapeutic data were available in 55 patients, and 39 (71%) received chemotherapy (primarily CHOP-like with or without RT), 4 had RT alone, and 12 (22%) were observed. With a median follow-up of 2 years (range, 0.1 to 14 years), the 5-year OS in patients with a breast mass was inferior to that in patients without a mass (100% vs 75%; P = .0308).
Most patients with BIA ALCL present with an isolated effusion, and removal of the implant and capsule results in excellent outcomes. Conversely, patients presenting with a breast mass may have a more aggressive course that would justify chemotherapy in addition to implant removal; however, the precise role for chemotherapy is uncertain.
Relapsed or refractory ALCL
Role of transplantation
HDC and ASCT represent the standard of care for relapsed ALCL if chemosensitivity is demonstrated. The phase 3 PARMA RCT established the superiority of HDC/ASCT over salvage therapy alone, and subsequent analyses of prognostic factors showed no difference in OS by T-cell vs B-cell phenotype; however, only 35 patients had T-cell NHL, and ALCL was not yet recognized.57 Although retrospective in nature, there have been numerous other studies evaluating the efficacy of ASCT in relapsed PTCLs that report 3- and 5-year EFS rates ranging from 25% to 75%,58 with some studies demonstrating salvage rates comparable to those seen in DLBCL, especially for patients with ALCL.59-62
The good salvage rates for patients with relapsed/refractory ALCL who receive HDC/ASCT were also observed in a Centre for International Blood and Bone Marrow Transplant Research63 study that evaluated 241 patients with PTCL (112 ALCL, 14 ALK+, 8 ALK−, 90 ALK status unknown) who had undergone either an ASCT or allogeneic SCT (alloSCT). In total, 61 patients with ALCL who received an HDC/ASCT were included, 39 of whom were beyond CR1. For the latter group, the 3-year PFS was 50% and the 3-year OS was 65%. In stark contrast to these studies, one report evaluating the impact of ASCT in 16 patients with relapsed/refractory ALK− ALCL demonstrated dismal outcomes, with a median PFS of only 12 weeks.64 The reason for the discrepancy is unclear, but the latter study could have included patients with CD30+ PTCL-NOS or patients who were enriched for early relapses.
Information is more limited on the role of alloSCT in relapsed/refractory ALCL, and many studies pool all PTCL subtypes. Taken together, myeloablative alloSCT in this setting results in approximately 30% of patients remaining alive and disease-free at 3 to 5 years with a full myeloablative transplantation; however, treatment-related mortality (TRM) rates are also ∼30%, and very few studies have reported results for ALCL.58,63,65,66 The Centre for International Blood and Bone Marrow Transplant Research study demonstrated that ASCT was associated with a better PFS (55% vs 35%; P = .0319) and OS (68% vs 41%; P = .0034) compared with alloSCT if all ALCL patients were considered.63 Restricting the analysis to patients beyond CR1 showed a superior 3-year OS for ASCT (62% vs 33%; P = .0088) but no difference in PFS. A separate retrospective analysis of 77 patients with PTCL who received an alloSCT demonstrated a 5-year EFS of 48% and 5-year OS of 55% for patients with ALCL (n = 27), but this study included patients who had received only 1 line of chemotherapy prior to alloSCT.65 A subset of PTCL patients with either stable or progressive disease at the time of transplantation benefited from alloSCT, with a 5-year OS of 29%, suggesting there may be a role in refractory ALCL.65 With the high TRM of myeloablative alloSCT, several studies have explored reduced-intensity conditioning in relapsed/refractory PTCL. A phase 2 trial evaluating reduced-intensity conditioning and alloSCT in 17 relapsed/refractory PTCL patients (4 ALK− ALCL), demonstrated a 3-year PFS of 64% with a TRM of 6%, suggesting it may have a role in select circumstances.67
In relapsed/refractory ALCL patients ineligible for transplantation or for whom second-line salvage therapy has failed, the outcome has historically been poor. The British Columbia Cancer Agency evaluated the survival of PTCL patients following first relapse or progression who had received chemotherapy, and the median OS and PFS were only 3.0 months and 1.8 months, respectively, for patients with ALCL, which supports a role for novel therapies and clinical trials for this poor-risk group.
Novel therapies in systemic ALCL
There has been an unprecedented number of trials evaluating novel therapies in relapsed/refractory PTCLs. Most have included all PTCL subtypes, but there has been a minority specifically in systemic ALCL. The antibody drug conjugate brentuximab vedotin (SGN-35) is the most widely studied agent in ALCL. It is composed of an anti-CD30 antibody conjugated by a protease-cleavable dipeptide linker to the anti-microtubule agent monomethyl auristatin E. After the antibody drug conjugate binds to CD30, the complex is internalized and monomethyl auristatin E is released by proteolytic cleavage to exert its cytotoxic effect. A phase 2 study in relapsed/refractory ALCL (42 ALK−, 16 ALK+)68 demonstrated an overall response rate (ORR) of 86% and CR of 57%. The estimated median PFS was 13.3 months, and for those who achieved a CR, it was 14.6 months. The most notable side effect was peripheral sensory neuropathy occurring in 41% with 12% considered grade 3. On the basis of these data, brentuximab vedotin was approved by the FDA in 2012 for relapsed/refractory ALCL following 1 line of therapy. A subsequent analysis of patients observed for almost 3 years demonstrated a median duration of response for CR patients of 26.3 months, and 16 (47%) of 34 remained in remission.69 In addition, the median PFS had not yet been reached for those who received an SCT (8 ASCT, 9 alloSCT) and was 18.4 months for those who did not. Interestingly, the efficacy of brentuximab vedotin is much less striking in CD30+ PTCL-NOS with an ORR of 33% (CR, 17%) and a median duration of response of 7.6 months, but the overall median PFS was only 1.6 months, which highlights that these are different diseases.70
Brentuximab vedotin was recently evaluated in the first-line setting in CD30+ PTCLs, including ALCL, either as a sequential treatment of 2 cycles followed by CHOP or in combination with CHP (with vincristine removed because of overlapping neurotoxicity).71 Responders could receive 8 to 10 additional cycles. The majority of patients (32) had ALCL (6 ALK+, 26 ALK−). Considering all CD30+ PTCL patients (n = 39), the ORR was 85% (CR, 62%) for the sequential therapy and 100% (CR, 88%) for the combination treatment; no patients received a consolidative ASCT. With a median follow-up of 21 months, 9 of 19 patients with ALCL had progressive disease or they died. The median PFS and OS have not been reached. These data form the basis of the ongoing ECHELON-2 phase 3 RCT (NCT01777152), which compares standard CHOP to CHP and brentuximab vedotin in newly diagnosed CD30+ PTCLs.
Crizotinib is an oral ALK inhibitor that has been explored in ALK+ ALCL. A phase 1 pediatric dose-escalation study included 9 patients with relapsed/refractory ALK+ ALCL, 7 of whom responded.72 Although data are more limited in adult patients, a recent case series described 9 adults (age 19-55 years) with relapsed/refractory ALCL, all of whom had CRs following treatment with crizotinib, some of which are quite durable at more than 40 months.73
There have been several additional studies evaluating novel treatments more broadly in all PTCLs. The first FDA-approved drug in relapsed/refractory PTCLs was the anti-folate pralatrexate based on a phase 2 study in relapsed/refractory PTCL that included 17 patients with ALCL (11 ALK−, 4 ALK+, 2 unknown). The ORR was 29% (CR, 10%), with a median PFS of 3.5 months, and efficacy was similar in ALCL patients (ORR, 35%).74 There is also growing body of literature supporting a rationale for epigenetic therapies in PTCLs, including the activity of histone deacetylase inhibitors. Romidepsin was evaluated in a phase 2 study of 130 patients with relapsed/refractory PTCLs, and it demonstrated an ORR of 25% for all PTCLs, with a median PFS of 4 months, which led to FDA approval in this setting. The efficacy was comparable in ALK− ALCL (n = 21; ORR, 24%).75 Similarly, a phase 2 study has been completed that evaluated the efficacy of belinostat in 129 patients with relapsed/refractory PTCL, including 15 patients with ALCL. The ORR was 26%, with a median PFS of 1.6 months, but the results by PTCL subtype have not yet been reported.76
Preliminary studies have explored the efficacy of other agents in relapsed/refractory PTCL, including the aurora A kinase inhibitor alisertib (ORR, 24%)77 and the PI3K inhibitor duvelisib (IPI-145) (n = 15; ORR 47%).78 The PD1 inhibitor nivolumab is under evaluation in NHLs, including PTCLs.79 Several of the above therapies are also being explored in combination because of complementary antitumor effects.
Future directions
Recent insights into the genetic heterogeneity of ALK− ALCL will aid risk stratification and will provide critical prognostic information when comparing treatment strategies. The success of brentuximab vedotin in relapsed/refractory ALCL compares favorably to historically poor outcomes in this setting, and results from the first-line phase 3 study are eagerly awaited. Future research exploring genetic factors driving disease pathogenesis and biomarkers of treatment response will be key in the development of a more personalized approach to treatment of patients with systemic ALCL.
Acknowledgments
The authors thank Dr Randy D. Gascoyne for providing the hematoxylin and eosin and immunohistochemistry images for anaplastic large cell lymphoma and peripheral T-cell lymphoma, not otherwise specified and Drs Randy D. Gascoyne and Graham W. Slack for their expert review of this manuscript.
Authorship
Contribution: G.H. and K.J.S. wrote and approved the manuscript.
Conflict-of-interest disclosure: K.J.S. has received honoraria from Seattle Genetics, Celgene, and Bristol Meyers Squibb. The remaining author declares no competing financial interests.
Correspondence: Kerry J. Savage, British Columbia Cancer Agency, 600 West 10th Ave, Vancouver, BC Canada; e-mail: ksavage@bccancer.bc.ca.