Abstract
Juvenile myelomonocytic leukemia (JMML) is a unique, aggressive hematopoietic disorder of infancy/early childhood caused by excessive proliferation of cells of monocytic and granulocytic lineages. Approximately 90% of patients carry either somatic or germline mutations of PTPN-11, K-RAS, N-RAS, CBL, or NF1 in their leukemic cells. These genetic aberrations are largely mutually exclusive and activate the Ras/mitogen-activated protein kinase pathway. Allogeneic hematopoietic stem cell transplantation (HSCT) remains the therapy of choice for most patients with JMML, curing more than 50% of affected children. We recommend that this option be promptly offered to any child with PTPN-11-, K-RAS-, or NF1-mutated JMML and to the majority of those with N-RAS mutations. Because children with CBL mutations and few of those with N-RAS mutations may have spontaneous resolution of hematologic abnormalities, the decision to proceed to transplantation in these patients must be weighed carefully. Disease recurrence remains the main cause of treatment failure after HSCT. A second allograft is recommended if overt JMML relapse occurs after transplantation. Recently, azacytidine, a hypomethylating agent, was reported to induce hematologic/molecular remissions in some children with JMML, and its role in both reducing leukemia burden before HSCT and in nontransplant settings requires further studies.
Clinical case
A 26-month-old boy was referred to the Pediatric Department because of fever, lymphadenopathy, facial skin rash, abdominal distention, bruising, and pallor. The spleen and liver were palpable 5 and 3 cm below the costal margin, respectively. Complete blood cell count showed leukocytosis (58 × 109/L), thrombocytopenia (29 × 109/L), anemia (7 g/dL), and striking monocytosis (3.95 × 109/L); dysplastic monocytes were observed on peripheral blood smear evaluation. The bone marrow (BM) aspirate revealed hypercellular marrow, with 7% blast cells. No bcr/abl fusion transcript was found and karyotype on BM cells was 46,XY. A diagnosis of juvenile myelomonocytic leukemia (JMML) was suspected and then confirmed by detection of a somatic N-RAS mutation. Because no family HLA-identical donor was available, a search for either a suitable unrelated volunteer or suitable cord blood (CB) unit was started. A 5/6 HLA-matched CB unit was located, and 3 months after diagnosis the patient received umbilical CB transplantation (UCBT) after a busulfan-based myeloablative regimen. After initial detection of complete donor chimerism, mixed chimerism with 10% recipient cells was documented on day +51 during tapering of cyclosporine (Cs-A), which had been administered for graft-versus-host disease (GVHD) prophylaxis. CsA was immediately discontinued, and 10 days later the child developed grade II acute GVHD, which resolved with steroid therapy. Complete donor chimerism was detected from day +90 after UCBT, and 3 years after transplantation the child remains disease free.
This case illustrates some of the typical diagnostic/therapeutic features related to this rare myeloproliferative disease of early childhood. Indeed, JMML is characterized by overproduction of monocytic and granulocytic cells that infiltrate different organs, including the spleen, liver, lung, and gastrointestinal tract, where diarrhea—sometimes with bloody features—may occur.1-4 Affected children usually have pallor, fever, and skin bleeding, which result from anemia, leukocytosis, and thrombocytopenia. In contrast to Philadelphia-positive chronic myeloid leukemia, the white blood (WBC) count rarely exceeds 100 × 109/L, the median value reported in a large cohort of patients being 33 × 109/L (range 5-259 × 109/L).2 A WBC <10 × 109/L at diagnosis is sometimes noted, particularly in children with monosomy 7.2
Morphologic evaluation of peripheral blood smear is the most important step in establishing the diagnosis. Immature monocytes, along with myelocytes, metamyelocytes, and erythroblasts, are usually found.1,2,5,6 Almost all cases show striking monocytosis, with dysplastic cells; an absolute monocyte count >1 × 109/L is required for diagnosis of JMML.4,5,7,8 A remarkable feature of many JMML cases with normal karyotype is a markedly increased synthesis of fetal hemoglobin (HbF).9 BM findings in JMML are not solely diagnostic, but are rather consistent with the diagnosis. BM aspirate shows hypercellularity with predominance of granulocytic cells at all stages of maturation, although sometimes erythroid series predominates: blast percentage is moderately elevated but never reaches the level seen in acute leukemia.
Chromosomal studies of leukemic cells show monosomy 7 in approximately 25% of patients with JMML, other abnormalities being found in another 10% of children; however, the majority of cases (65%) have a normal karyotype.2,3,5,10 The frequency of monosomy 7 among the genetic subtypes varies, being in our experience more frequently detected in children with K-RAS anomalies.
JMML may have an incidence of as much as 1.2 per million children per year, accounting for 2% to 3% of all childhood hematologic malignancies.11 Median age at presentation is 2 years (range 0.1-11.4) and males are more frequently affected (male:female ratio 2-3:1).2 To account for both the myelodysplastic and proliferative features noted in JMML, the World Health Organization (WHO) classification placed the entity in the group of myelodysplastic/myeloproliferative disorders.12 Diagnosing children with JMML is sometimes challenging,13 because the clinical picture of JMML can be mimicked by a number of human herpesvirus infections,14 leukocyte-adhesion deficiency, infantile malignant osteopetrosis, hemophagocytic lymphohistiocytosis, and Wiskott-Aldrich syndrome.5,15 The recent discovery that approximately 90% of children with JMML have either somatic or germline mutations in genes involved in the RAS signaling pathway (discussed later), in addition to greatly improving our understanding of the molecular pathogenesis of this disorder, has enormously facilitated diagnosis by allowing mutational analyses. Thus it is not surprising that the criteria initially proposed and used for many years for diagnosing JMML8 have been revised over time.4,5,7 The most recent criteria by which oncogenetic features largely predominate and that we currently use to diagnose children with JMML are reported in Table 1.
Pathophysiology and genetics of JMML
Hypersensitivity of JMML myeloid progenitor cells to granulocyte-macrophage colony-stimulating factor (GM-CSF) and pathologic activation of the RAS-RAF-MAPK (mitogen-activated protein kinase) signaling pathway play an important role in disease pathophysiology.16,17 Indeed, GM-CSF hypersensitivity of monocyte/macrophage colonies is a hallmark of the disease, and for many years represented an important diagnostic tool.18,19 This peculiar GM-CSF hypersensitivity can still be used as a diagnostic criterion for JMML for those few patients without a detectable known molecular lesion (Table 1). However, GM-CSF hypersensitivity has been reported also to be induced by HHV-620 and cytomegalovirus infections,21 and the assay is not standardized among laboratories. Recently, hyperphosphorylation of STAT5 in response to GM-CSF documented through phosphospecific flow cytometry in either CD33+/CD34+ or CD33+CD14+CD38low cells has been found in a proportion of patients with JMML,22,23 and thus could also be helpful in supporting the diagnosis of children without any detectable molecular lesion (Table 1).
As was already mentioned, major progress in understanding the pathogenesis of JMML has been achieved in the last 2 decades by mapping out the underlying genetic lesions.4,10,16,24 This molecular characterization was certainly facilitated or even promoted by the discovery of a group of genetic syndromes resulting from germline mutations in genes of the RAS/MAPK pathways. These mutations induce the activation of the pathway, and thus these disorders, collectively grouped as “neuro-cardio-facio cutaneous syndromes (NCFCS)” or “RASopathies,” share common clinical features, including a propensity to develop malignancies, among which myeloproliferative disorders are relevant.25,26 Neurofibromatosis type 1 (NF-1) and Noonan syndrome (NS), caused by mutations of NF1 in the former and of PTPN-11 in half of the cases in the latter, are the most frequent and known.25-27 A transient myeloproliferative disorder (TMD) is diagnosed in a proportion of NS children in the neonatal period/early infancy. In contrast to JMML, NS/MPD generally resolves spontaneously over months; thus a “watch and wait” strategy is appropriate for these children.4,25 These infants with NS very often also have cardiac anomalies, and life-threatening or even fatal complications at least in part caused by leukocytosis and tissue invasion by monocytes and immature granulocytes may develop in a certain proportion of them. We recommend that this subset of NS patients be treated with mild cytoreductive therapy, such as 6-mercaptopurine. Moreover, because some cases of NS/MPD acquire cytogenetic abnormalities and progress to classically aggressive JMML, we recommend also that these patients be followed closely to timely diagnose and treat possible evolution.
The spectrum of mutations described thus far in JMML occurs in genes all encoding proteins that signal through the RAS-RAF-MAPK pathways.10 These genes include NF1, N-RAS, K-RAS, PTPN-11, and, as most recently demonstrated, CBL.10,16,28 Although largely mutually exclusive, mutations in NF1, PTPN-11, K-RAS, N-RAS, and CBL are detected in 90% of patients with JMML.16,24 Specifically, ∼35% of patients with JMML carry somatic mutations in PTPN-11,29,30 20% to 25% carry aberrations in N-RAS or K-RAS,31-33 and 11% of patients with JMML were diagnosed with clinical NF-1.2,4,34 Because heterozygous point mutations noted in PTPN-11 and RAS may arise at a somatic or germline level, we recommend that genetic screening in leukemic cells be followed by studies in nonhematopoietic tissue, such as fibroblasts, cells attached to nails, or hair bulbs. The reason for this recommendation lies on the observation that, besides those with NS and germline PTPN-11 mutations, the rare children carrying germline N-RAS and K-RAS mutations and presenting myeloproliferation generally show amelioration with time and thus do not require HSCT (Table 2).35
Patients carrying the CBL mutation (found in as much as 15% of all cases) deserve a particular mention, because they display several congenital anomalies that overlap with those observed in NF-1, NS, and Legius syndrome.28,36,37 These children have germline CBL mutations and JMML develops because they are at increased risk of developing loss of heterozygosity (LOH) for the CBL locus in hematopoietic stem/progenitor cells. Indeed, all children with JMML and CBL mutations were found to have a germline CBL missense mutation on 1 allele and acquired LOH on the other allele in leukemic cells. The resulting myeloproliferative disorder is often self-resolving, although these children may have an aggressive clinical course (Figure 1).28,36,38,39 Some children with germline CBL mutations and JMML had vasculitis later in life. Similarly, mice that lacked Cbl had severe vascular lesions with massive infiltration of T cells and high concentrations of anti–double-stranded DNA antibodies.40
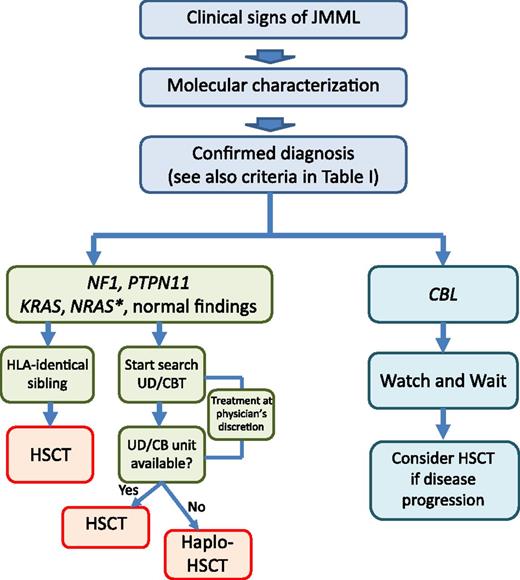
Algorithm for treatment of children with JMML, stratified according to somatic (N-RAS, K-RAS, PTPN-11) or germline (CBL, NF1) molecular lesions and availability of an HLA-identical sibling. *In some patients with somatic N-RAS mutations (ie, those with low HbF and high platelet count), long-term survival in the absence of allogeneic HSCT has been observed. Although it is still an experimental therapy for JMML, haploidentical HSCT is an option in the few patients who need an urgent allograft and lack any suitable HLA-identical sibling, adult UD, or unrelated CB unit.
Algorithm for treatment of children with JMML, stratified according to somatic (N-RAS, K-RAS, PTPN-11) or germline (CBL, NF1) molecular lesions and availability of an HLA-identical sibling. *In some patients with somatic N-RAS mutations (ie, those with low HbF and high platelet count), long-term survival in the absence of allogeneic HSCT has been observed. Although it is still an experimental therapy for JMML, haploidentical HSCT is an option in the few patients who need an urgent allograft and lack any suitable HLA-identical sibling, adult UD, or unrelated CB unit.
Although mutations in NF1, PTPN-11, K-RAS, N-RAS, and CBL represent the most prominent genetic feature of JMML known thus far, it is still unclear how they determine treatment resistance. Alterations of genes, such as JAK2, TET2, RUNX1, and ASXL1, seen in other myeloproliferative disorders of adulthood, are rare.37,41,42 Recently, through an exome-sequencing approach, it was confirmed that JMML is characterized by a paucity of gene mutations. Secondary mutations of SETBP1 and JAK3 were the most frequent mutations, detected in around 15% of children.43 These mutations of SETBP1 and JAK3 were presumed to be involved in tumor progression and were associated with poor clinical outcomes.43 Recently, a study based on the use of droplet digital polymerase chain reaction (ddPCR) identified SETBP1 mutations in 17 of 56 (30%) children with JMML.44 Also in this study, SETBP1 mutations were associated with a dismal prognosis.
Should all children with JMML be immediately offered allogeneic HSCT?
In the vast majority of cases, JMML is an aggressive and fatal disorder if left untreated; the median survival time of children who do not receive an allograft can be as short as 10 to 12 months.2 Blastic transformation is infrequent in JMML, and most untreated patients succumb to respiratory failure as a result of pulmonary infiltration of leukemic cells. Clinical risk assessment in JMML includes age at diagnosis, platelet count, and percentage of HbF adjusted for patient age as main prognostic variables.2,6 In particular, in a large cohort of patients with JMML, age >2 years at diagnosis, platelet count <33 × 109/L, and levels of HbF ≥10% were the main predictors of short survival.2,6 British investigators devised a scoring system, where HbF ≥10% and platelet count ≤33 × 109/L negatively affect prognosis.6
Recent studies have highlighted the importance of epigenetic aberrations (aberrant DNA methylation) in JMML.45,46 In particular, we investigated the association of CpG island hypermethylation in JMML leukemic cells with known clinical risk factors.45 There was a strong association between CpG island hypermethylation and older age, elevated HbF at diagnosis, and a particularly dismal outcome. PTPN-11–positive cases were overrepresented in the hypermethylated group, whereas all CBL-mutant cases belonged to the group with absent or low hypermethylation.45 A more recent study also documented that methylation of the RASA4 isoform–2 promoter correlated with clinical parameters predicting poor prognosis (older age, elevated fetal hemoglobin), with PTPN-11 mutation, and higher risk of relapse after HSCT.46
Allogeneic HSCT is the firmly established curative approach for most children with JMML, resulting in long-term survival in many patients47-52 ; thus, we recommend swift HSCT for all children with JMML and NF-1, somatic PTPN-11 mutations, and K-RAS mutations, and for the vast majority of children with somatic N-RAS mutations (Figure 1 and Table 2). As was already mentioned, children with germline CBL mutations and JMML often have spontaneous regression of their myeloproliferation despite the persistence of LOH of CBL in hematopoietic cells. In particular, an international study on CBL germline mutation reported that of the 6 patients with JMML and CBL mutation who did not receive HSCT, 4 were alive without evidence of JMML 7.5 to 18 years from diagnosis.28 Considering the 23 children with CBL-mutated JMML now registered into the European Working Group of MDS in Childhood (EWOG-MDS) database, the probability of survival was the same for children who did (12 children) and those who did not (11 children) receive HSCT, because 3 children in each group died. We therefore recommend that children with JMML and CBL germline mutations be followed closely and not be offered HSCT immediately after diagnosis. Transplantation must be considered, however, if chromosomal aberrations occur or if disease progresses (Figure 1). Intriguingly, in children with CBL mutation transplanted before the identification of this molecular lesion as responsible for the disease, a high rate of stable mixed chimerism was observed. None of these children with mixed chimerism is currently known to have vasculitis; whether HSCT can be useful to prevent vascular complications remains to be definitively proved.
In a small number of infants with RAS alterations, long-term survival in the absence of HSCT has been observed.53,54 In a genotype-phenotype correlation study, Matsuda et al suggested a mild clinical course for the G12S substitution in N-RAS or K-RAS protein.53 Although data from EWOG-MDS confirmed that sporadic patients with RAS-mutated JMML may enjoy long-term survival without intervention, they did not support an overrepresentation of a specific mutational RAS type in cases with this less aggressive course.55 In our experience, children with RAS-mutated JMML who survived without transplantation had normal HbF and high platelet count.55 Thus, so far, in the absence of robust biological markers that are able to predict spontaneous resolution of JMML, we advise that these simple hematologic parameters be used to identify those few children with RAS-mutated JMML in whom a “watch and wait” strategy can be adopted (Figure 1).
Results obtained with HSCT in JMML and factors influencing outcome
The outcome of patients with JMML given HSCT has progressively improved over time. In the study including the largest number of patients with JMML given allogeneic HSCT, either from a histocompatible relative or from an HLA-matched/1-antigen-disparate unrelated donor (UD), the probability of disease-free survival (DFS) was 52% (95% confidence interval [CI] 42-62).48 Disease recurrence was the most important cause of treatment failure, occurring with a cumulative incidence of 35% (95% CI 27-46).48 In multivariate analysis, age >4 years and female sex predicted poorer outcome. In a Japanese study including 27 children with JMML, the probability of DFS was reported to be 54% ± 11%.50 Although in initial reports of HSCT in JMML, the probability of transplantation-related mortality (TRM) for children transplanted from matched UDs/mismatched relatives was worse than that of children given the allograft from an HLA-identical/1-antigen-disparate relative,47,56 in more recent years, using an UD offers minimal or no significant disadvantage compared with using an HLA-identical sibling.48,51
Available data indicate that UCBT is a suitable option for children with JMML lacking an HLA-compatible relative.57-59 Two robust arguments for considering UCBT in children with JMML are that these patients frequently need urgent transplantation and their body weight is low. The short time interval elapsing between start of the search for locating an UD and identification of a suitable CB unit renders UCBT an attractive option for patients with JMML.15 Different studies have shown an inverse correlation between the number of CB cells infused per kg of the recipient’s body weight and the risk of TRM.60-62 Because >90% of children with JMML are diagnosed before 4 years of age,2 it is evident that a favorable ratio between total nucleated cells infused and the recipient’s body weight can be obtained in the large majority of patients. A recent retrospective analysis on 110 children with JMML given single-unit unrelated UBCT demonstrated outcomes similar to that reported with different donors or sources of hematopoietic stem cells, the 5-year Kaplan-Meier estimate of DFS being 44% ± 5%.57 Despite the relevant HLA disparity in many of the donor/recipient pairs, the probabilities of grades II-IV acute and chronic GVHD in UCBT were comparable with those reported in studies using HLA-matched bone marrow/peripheral blood cells.57 There was a trend for lower TRM in patients given UCBT after 2003 than in those transplanted before 2003 (16% vs 29%, respectively). Disease recurrence was the major cause of treatment failure also in children given UCBT, the reported cumulative incidence of relapse being 33%. Children transplanted from a donor with 0/1 HLA disparity were found to have better outcome compared with those transplanted from donors with greater disparities,57 this finding being in keeping with previously published reports showing a clear advantage for children transplanted using HLA-matched units.61,63 Thus, we recommend that in JMML children lacking a compatible sibling, the search for locating either an UD or a suitable CB unit be started simultaneously (Figure 1) and that, whenever possible, units with limited HLA disparity with the recipient be selected.57 Moreover, because a high number of cells infused per kilogram of recipient body weight predict a better outcome, we recommend that CB units with at least 3-3.5 × 107 nucleated cells per kg of recipient body weight before thawing be selected.59,64
Although HSCT from an HLA-haploidentical relative is still “experimental” in JMML, it can be considered for those few patients in need of an urgent allograft who lack any suitable HLA-identical sibling, adult UD, or unrelated CB unit (Figure 1).
The relapse rate in children with JMML given HSCT has been reported to be as high as 40% to 50%.48,50,57 Age >4 years, as well as a BM blast percentage >20%, has been found to be associated with an increased risk of recurrence.48 Although 1 study reported a negative impact of monosomy 7 on the probability of overall survival (OS) after HSCT,50 other larger analyses documented that neither monosomy 7 nor other cytogenetic abnormalities confer a poorer prognosis.48,57 Recently, Bresolin et al reported that gene expression signatures could segregate JMML patients into those who displayed an acute myeloid leukemia–type signature vs those who did not.65 In multivariate analysis, children who displayed an acute myeloid leukemia–type signature had an increased risk of recurrence after HSCT. High methylation of CpG islands in some genes of children with JMML has also been recently reported to predict an increased risk of recurrence after HSCT.45 Some subjects showed a more hypermethylated phenotype at time of relapse when compared with their methylation status at diagnosis.45
The existence of a correlation between the JMML mutational status and outcome of patients given HSCT remains controversial. In this respect, Yoshida and colleagues evaluated the clinical course and laboratory findings of 49 children with JMML, 32 of whom harbored mutations in NF1, K-RAS, N-RAS, or PTPN-11.66 In their series, mutation of PTPN-11 was associated with older age at diagnosis (>24 months) and increased HbF (>10%) and, importantly, it appeared to be an unfavorable prognostic factor predicting relapse after HSCT.66
How to optimize the transplant approach in children with JMML
Preparative regimens that do not include total body irradiation (TBI) are particularly attractive for children with JMML, because radiation-induced late effects may be especially deleterious for very young children.67-70 Moreover, in a retrospective EWOG-MDS analysis, busulfan-based myeloablative therapy offered greater antileukemic efficacy than TBI.47 Thus, we advise that children with JMML are prepared for HSCT using a myeloablative regimen based on the combination of busulfan with other cytotoxic drugs (Figure 2). The preparative regimen used by the EWOG-MDS group includes busulfan, cyclophosphamide, and melphalan.48,57 The rationale for this choice relies on the consideration that a preparative regimen consisting of 3 alkylating drugs that have non-cell-cycle–specific action appears potentially capable of eradicating stem cell disorders, such as JMML, in which a portion of clonogenic cells are dormant out of cell cycle. Japanese investigators recently reported on the use of a preparative regimen consisting of busulfan, fludarabine, and melphalan in an attempt to decrease TRM and reduce the risk of graft failure.49 Although the number of patients reported in this study is much smaller than that of the EWOG-MDS group, at the time of reporting, 7 of 10 patients transplanted were alive and in remission, with a median follow-up of 30 months. In the recent retrospective analysis on 110 children with JMML given single-unit unrelated UBCT, patients given either the preparative regimen recommended by the EWOG-MDS group or another busulfan-based myeloablative therapy had similar outcome.57 The Children’s Oncology Group (COG) is currently prospectively comparing the preparative regimen including busulfan, cyclophosphamide, and melphalan vs a combination of busulfan and fludarabine, to test the hypothesis that the latter regimen is associated with both less TRM and comparable DFS.
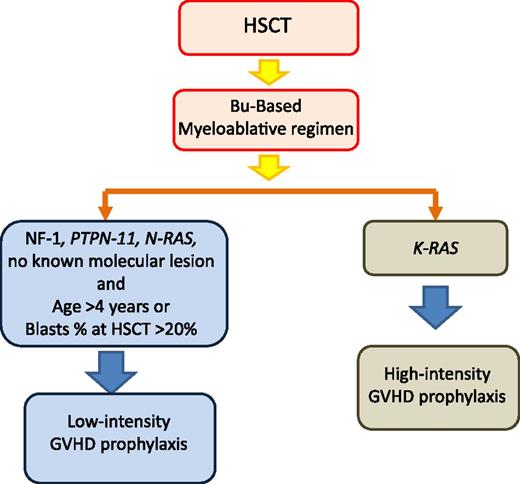
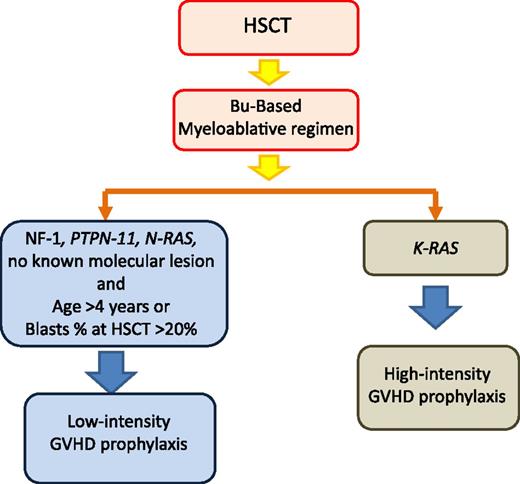
Tailoring of GVHD prophylaxis according to patient’s genetic lesions and other risk factors influencing the relapse rate. HSCT, hematopoietic stem cell transplantation; Bu, busulfan.
Tailoring of GVHD prophylaxis according to patient’s genetic lesions and other risk factors influencing the relapse rate. HSCT, hematopoietic stem cell transplantation; Bu, busulfan.
Serotherapy (such as antithymocyte globulin [ATG]) is often included in the conditioning regimen when an UD, either of BM, peripheral blood, or CB, is used. Despite theoretic concerns that serotherapy may potentially reduce in vivo antileukemia alloreactivity, the available evidence indicates that its use does not increase the relapse rate of children with JMML.48,57 By contrast, ATG may contribute to reduce the risk of fatal complications related to GVHD. Thus, we believe that ATG should be used in children with JMML given an UD HSCT or UCBT.
Incidence of both acute and chronic GVHD in patients with JMML reported in different studies tend to be lower than in other leukemic diseases,48,50,57 this possibly being related to the young age of patients with JMML, and GVHD did not represent a major cause of TRM. In the study of children with JMML given UCBT, decreased incidence of relapse was associated with the presence of grades II-III acute GVHD.57 Moreover, investigators from the United States and Japan noted better survival for children who did develop chronic GVHD as compared with those who did not.50,51 These findings suggest the existence of a graft-versus-leukemia (GVL) effect directed against JMML cells with subsequent protection against relapse. Support to this hypothesis is given by the observation that withdrawal of immunosuppressive therapy in patients with incipient relapse of JMML can prevent the subsequent occurrence of overt relapse.71-73 In view of these considerations, we recommend that JMML children with NF-1, somatic PTPN-11, or N-RAS mutations, age >4 years at time of diagnosis or with >20% of blasts at the time of HSCT, receive low-intensity GVHD prophylaxis with the aim of optimizing the GVL effect (Figure 2). In the absence of acute GVHD, prophylaxis should be discontinued between day +60 and +90 after HSCT. A remarkable exception to this recommendation is represented by children carrying K-RAS mutations because, in our experience, these children have a lower relapse rate than children with other molecular abnormalities (Figure 2).
Gross spleen enlargement is usually found in many children with JMML. Pretransplantation splenectomy has been used in several children with JMML,47,74 also with the hope of promoting donor cell engraftment and of reducing tumor burden at the time of HSCT, thus potentially translating into a reduced risk of recurrence. However, splenectomy before HSCT, as well as spleen size at the time of the allograft, was not found to have any impact on posttransplantation outcome of children with JMML.48 Thus, available data are not in favor of indiscriminate use of splenectomy before transplantation, the potential advantages having to be weighed against the risks related to the procedure or to postsplenectomy infections.75 The presence of massive splenomegaly with evidence of hypersplenism and/or refractoriness to platelet transfusions could be arguments for considering this procedure in selected cases.
There is no doubt that a relevant area of controversy surrounding the care of JMML patients also concerns the role of antileukemic therapy before transplantation. The need, or even the opportunity, for treating JMML children with conventional chemotherapy is currently uncertain and the comparative evaluation of different clinical protocols has been hampered by lack of uniform criteria for response.76 To date, no standard chemotherapy regimens used before HSCT have been shown to have a real impact on the incidence of post-HSCT relapse. In some patients, a decrease in leukocytosis and spleen size can be noted with oral 6-mercaptopurine and/or low-dose cytarabine. In children with blastic transformation or life-threatening pulmonary infiltration, fludarabine and high-dose cytarabine may give temporary relief. “Watchful waiting” can be considered for those few patients with JMML who are asymptomatic. Overall, the pretransplant therapy is still a matter of controversy, and firm recommendations cannot be provided (Figure 1).
How we manage children with disease recurrence after an allograft
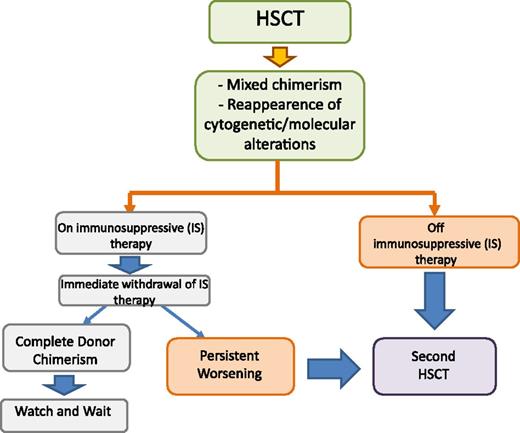
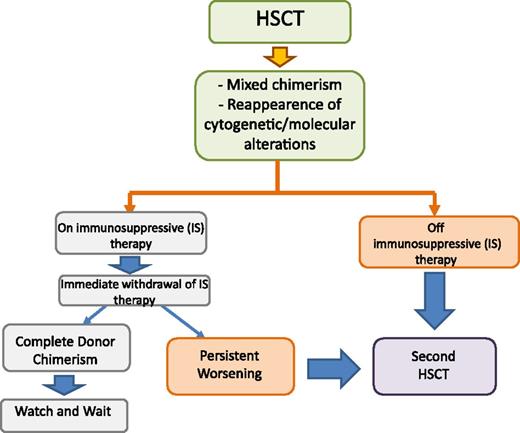
The algorithm that we use for treating either incipient or overt leukemia relapse in patients who are either still receiving or no longer receiving any immune-suppressive therapy is reported in Figure 3. Serial quantitative chimerism studies using short tandem repeat (STR) markers have been shown to be useful for identifying JMML patients with increasing mixed chimerism who are therefore at high risk for relapse.71,73 In these patients, immediate withdrawal of ongoing GVHD prophylaxis can lead to eradication of malignant cells regrowing and/or persisting after the conditioning regimen.71-73 However, although effective, this strategy can lead to the development of chronic GVHD in some patients.77 More sensitive methods to detect reemergence and/or persistence of malignant cells, such as those relying on disease-specific markers, may allow identification of a smaller percentage of residual leukemic cells, potentially increasing the chance of response to GVHD prophylaxis withdrawal.72 For this purpose, a fluorescent-based, allele-specific polymerase chain reaction assay for detecting the most common RAS or PTPN-11 mutations has recently been developed.78 Prospective studies aimed at validating the clinical benefit deriving from this approach are needed.
Algorithm for treatment of mixed chimerism and leukemia relapse in JMML in patients either still receiving or not receiving any immune-suppressive therapy. HSCT, hematopoietic stem cell transplantation.
Algorithm for treatment of mixed chimerism and leukemia relapse in JMML in patients either still receiving or not receiving any immune-suppressive therapy. HSCT, hematopoietic stem cell transplantation.
For children with JMML who have overt leukemia relapse after allogeneic HSCT, donor lymphocyte infusion (DLI) proved to be largely ineffective,71,79 whereas a second allograft from either the same or a different donor, together with reduction of the intensity of GVHD prophylaxis aimed at optimizing the GVL effect, was able to rescue more than one-third of patients.71,77,80 The biological/immunologic reasons why JMML recurrence has limited sensitivity to DLI remain obscure.72,79 Because a second HSCT can be an effective salvage therapy for children with JMML who relapse after a first grafting procedure,71,77,80 we believe that children with JMML should not receive DLI but are to be given a second transplant as soon as possible, especially if they have already discontinued GVHD prophylaxis.
What we envisage to be the role of innovative targeted drugs in JMML
So far, as already mentioned, therapies other than transplantation have had a limited role in children with JMML76 ; in addition, HSCT has some important limitations, including lack of efficacy in patients who relapse, TRM, and long-term sequelae. Available data indicate that the Ras/MAPK pathway is deregulated in JMML through not only genetic but also epigenetic changes.45,46,81 Whether the use of epigenetic drugs, such as 5-azacytidine (a DNA methyltransferase–inhibiting azanucleoside assumed to reverse epigenetic dysregulation in malignant cells), or the use of agents targeting the Ras/MAPK pathway before HSCT may be beneficial is currently unknown. Furlan and colleagues reported a successful anecdotal case of azacytidine use in a child with JMML with both K-RAS mutation and monosomy 7.82 The clinical and hematologic response obtained with the drug was impressive; already after the first course, a response in spleen size and monocyte count was noted.82 After the fifth course, monosomy 7 disappeared, and the K-RAS mutation, present at diagnosis, became undetectable after 7 courses. After the eighth course, HSCT was performed, and 5 years after transplantation the child remains disease free. Since this case was reported, we observed clinical and molecular responses to 5-azacytidine in 3 of 9 JMML patients treated off label before transplantation (unpublished personal data). Despite its clinical activity, 5-azacytidine is not expected to be ultimately curative in JMML; however, it can be tested as window therapy with the aim of effectively reducing the burden of disease before HSCT. A phase 2 multicenter study is underway in Europe to explore this hypothesis.
Analogous studies evaluating the hypothesis of whether MEK inhibitors might ameliorate hematologic abnormalities in JMML are also worthy of consideration, because MEK inhibition abrogated the myeloproliferative disease in Nf-1- and Kras-mutant mice.83-86 In particular, in Kras-mutant mice, PD0325901, a highly selective pharmacologic inhibitor of MEK, was shown to: (1) correct the aberrant proliferation and differentiation of bone marrow progenitor cells, (2) induce a rapid and sustained reduction in leukocyte counts, (3) enhance erythropoiesis, and (4) prolong mouse survival.24 Future clinical studies aimed at testing whether treating children with JMML with MEK inhibitors before HSCT might improve their clinical status by reducing the morbidity caused by infiltration of organs with leukemic cells are warranted.
Acknowledgments
This work was partially supported by grants from the Associazione Italiana Ricerca sul Cancro (progetto speciale 5xmille); Progetti di Rilevante Interesse Nazionale 2010; Ministero dell’Istruzione, Università e della Ricerca; and Ministero della Salute (Ricerca Finalizzata 2010) and Istituto di Ricovero e Cura a Carattere Scientifico Ospedale Pediatrico Bambino Gesù (F.L.).
Authorship
Contribution: F.L. and C.M.N. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Franco Locatelli, University of Pavia, Department of Pediatric Hematology and Oncology, IRCCS Ospedale Pediatrico Bambino Gesù, Piazza Sant’Onofrio, 4. 00165 Rome, Italy; e-mail: franco.locatelli@opbg.net.
References
Author notes
F.L. and C.M.N. contributed equally to the writing of this manuscript.