Abstract
The etiology of pediatric venous thromboembolic disease (VTE) is multifactorial, and in most children, 1 or more clinical risk factors are present. In addition, inherited thrombophilic disorders contribute to the development of pediatric VTE. In this review, the role of inherited thrombophilic disorders in the development of pediatric VTE, as well as the benefits and limitations of thrombophilia testing, will be discussed.
Introduction
Venous thromboembolic disease (VTE) is a rare disease in childhood. The reported annual incidence ranges from 0.07 to 0.14 per 10 000 children and is estimated to be 5.3 per 10 000 hospital admissions.1-3 The incidence seems to be increasing over the past decades. Raffini et al reported a rise in the annual incidence of VTE of 70%, from 34 to 58 cases per 10 000 hospital admissions.4 This is confirmed by Boulet et al.5 This rise is likely caused by an increased awareness of the disease, progress in radiologic imaging, and increased survival of children with previously incurable diseases because of medical and surgical improvements.
In 2008, a systematic review and meta-analysis concerning pediatric VTE and inherited thrombophilia (ie, deficiencies of antithrombin [AT], protein C [PC] and protein S [PS], and factor V Leiden mutation [FVL] and the prothrombin mutation [PTM]) showed elevated thrombotic risks in children with these defects.6 In the current review, we discuss additional literature after 2008 in various pediatric patient groups and the usefulness of thrombophilia testing.
Methods
Inclusion and exclusion criteria
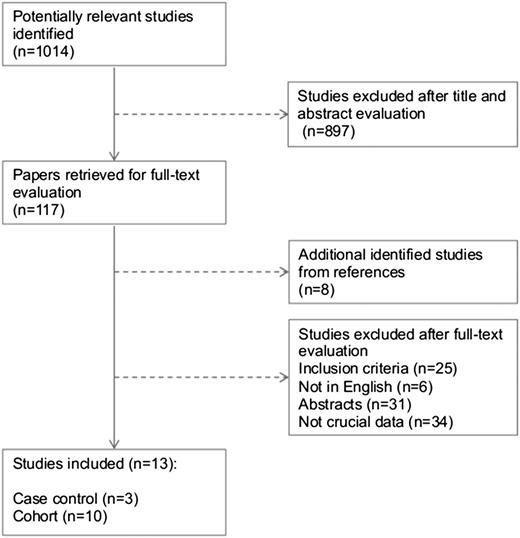
All full-text published studies of VTE and thrombophilia in neonates and children (0-18 years) from 2008 through October 2014 were evaluated for inclusion if (1) diagnosis of VTE of any location was objectively confirmed by accepted imaging methods; (2) frequency of at least one of the inherited thrombophilic factors was assessed in a given VTE cohort or the frequency of inherited thrombophilic factors was compared between VTE patients and controls without a history of VTE; and (3) inherited thrombophilic factors were measured in an accepted manner (Figure 1). Conference abstracts, case reports, and studies with unclear methodology to differentiate between inherited and acquired deficiencies of the natural anticoagulants were not included.
Search strategy
A systematic search of publications listed in the electronic databases MEDLINE, EMBASE, and The Cochrane Library from 2008 through October 2014 was conducted, with the limits set to only articles in the English language. The following key words were used in combination both as MeSH (medical subject heading) terms and text words: “thromboembolism or deep vein thrombosis or pulmonary embolism or venous thrombosis or anticoagulation or antithrombotic therapy” and “thrombophilia” or “hypercoagulat*” or “protein C” or “antithrombin” or “protein S” or “(factor 5 leiden or factor v leiden or fv leiden)” or “(factor 2 mutation or factor II mutation or prothrombin mutation)” and “adolescen* or child* or infan* or newborn* or neonat* or school child* or baby or babies or toddler* or prematur* or preterm* or vlbw or elbw.” Citations of the searched articles were included in the review if they were determined to be relevant. Data extraction was performed in duplicate by 2 authors (I.L.M.K. and C.H.v.O.).
Quality assessment
Quality assessment of case-control and cohort studies was performed using the Newcastle-Ottawa Scale.7
Results
Venous thrombosis and thrombophilia in neonates
Although the meta-analysis of Young et al showed that thrombophilia contributes to the development of neonatal VTE, it should be realized that neonates were underrepresented in this meta-analysis.6 The incidence of symptomatic VTE in neonates is 5.1 per 100 000 births, and ∼95% of neonatal VTE is associated with at least 1 clinical risk factor, usually a CVC.13,14 Studies concerning thrombophilia in neonates with CVC-related thrombosis are scarce. One study that was included in the meta-analysis of Young et al described 10 neonates with symptomatic CVC-related thrombosis. No deficiencies of AT, PC, or PS were detected. One patient was heterozygous for FVL.15 One study was performed after 2008. It showed that thrombophilia was present in none of the 13 studied neonates with CVC-related thrombosis.16
Renal vein thrombosis (RVT) is the most frequent noncatheter-related VTE in neonates, and its incidence was reported to be 2.2 per 100 000 births in Germany.17 The prevalence of inherited thrombophilia seems to be higher in neonates with RVT than with catheter-related VTE, but only a few small studies have been published, all before 2008. The meta-analysis of Young et al included 2 small studies. Marks et al retrospectively identified 43 neonates with RVT between 1980 and 2001 in 4 pediatric Canadian tertiary care centers. Twelve of the 28 neonates (43%) studied had thrombophilias.18 A case control study by Kosch et al reported an even higher prevalence: in 40 (67.8%) of the 59 neonates, at least 1 thrombophilia was present compared with 14 (11.9%) of the 118 controls (odds ratio [OR], 15.6; 95% confidence interval [CI], 7.2-34.2).19 In addition, this study confirmed that neonatal RVT is a multifactorial disease: several underlying clinical risk factors, such as asphyxia, sepsis, diabetic fetopathy, and CVCs, were present.
Neonatal CVT is a rare, but serious disease. In a multicenter, retrospective review by Berfelo et al, 2 (5%) of 41 neonates tested had FVL and 2 (11%) of 18 neonates tested had PTM.20 In a retrospective nationwide population-based study, 10 infants <1 year of age, including 7 neonates, were diagnosed with CVT. Inherited thrombophilia was present in only 1 (13%) of the 8 patients tested. Five (71%) out of 7 neonates had an underlying illness, such as asphyxia or sepsis.21 In 2010, a meta-analysis including both neonatal and childhood CVT showed statistically significant association between CVT and all inherited thrombophilic disorders, except PTM.22 In agreement, Laugesaar et al performed a meta-analysis separately for neonatal CVT, including their own study results, which showed a significant association between FVL and neonatal CVT (OR, 5.5; 95% CI, 2.1-14.5), but not between PTM and neonatal CVT (OR, 3.1; 95% CI, 0.8-12.4).23
In conclusion, only few studies investigated the prevalence and contributing role of thrombophilia in neonatal thrombosis. Neonatal VTE is a multifactorial disease, and clinical risk factors seem to play a more important role than inherited thrombophilia, especially in catheter-related VTE.
Thrombophilia in children
The meta-analysis by Young et al demonstrated that inherited thrombophilia contributed to the development of VTE in children.6 The ORs varied from 2.63 (95% CI, 1.61-4.29) for PTM to 9.44 (95% CI, 3.34-26.66) for AT deficiency. In children with ≥2 genetic traits, the combined OR was 8.89 (95% CI, 3.43-23.06).6 The observed relative risk (RR) estimates are similar to adults.24 Nevertheless, underlying clinical risk factors were present in a majority (>70%) of children with VTE. The most important risk factor in children is a CVC. However, other clinical risk factors, such as malignancy, cardiac disease, and NS, contribute to thrombotic risk as well.2
The presumed impact of inherited thrombophilia on the thrombotic risk of CVC-related VTE in children is challenged by results from 2 recent studies. Albisetti studied thrombotic events in children with malignancies and a PAC by magnetic resonance venography. Only 2 of 45 (4%) patients with CVC-related VTE had inherited thrombophilia compared with 8 of 69 (12%) patients without VTE.25 In addition, in the KIDs with Catheter Associated Thrombosis study, which included 90 children with heart disease requiring CVCs in the upper venous system for perioperative care, none of the thrombophilic factors showed a significant association with CVC-related VTE.26
VTE is one of the most serious complications in children with NS, which is attributed to a net shift in the hemostatic balance toward a hypercoagulable state by selective loss of hemostatic proteins, most notably PS and AT.25 The reported incidence of VTE in children with NS varies between 9% and 36% based on recent literature.27,28 Age ≥12 years at onset, severe proteinuria, and history of VTE prior to diagnosis of NS were significant independent predictors of VTE. The presence of thrombophilia was only evaluated in 12 of 30 VTE patients. In 4 patients (33%), FVL was identified.27 Suri et al retrospectively reviewed the clinical profile in nephrotic children with VTE. Hypoalbuminemia (83%) and infection (31%) were the most common predisposing factors. Two (11%) patients were diagnosed with inherited PS deficiency, and 1 patient was diagnosed with inherited AT deficiency (6%).28
In children, CVT has an annual incidence of 0.67 per 100 000.29 In 2010, a meta-analysis of Kenet et al, including 5 cohort studies with 297 patients with CVT found a statistically significant association between all inherited thrombophilic disorders, except PTM, and a first CVT onset in neonates and children.22 The summary ORs varied from 2.74 (95% CI, 1.73-4.34) for FVL to 18.4 (95% CI, 3.25-104.3) for AT deficiency. The study of Laugesaar et al was not included in the above-mentioned meta-analysis. They performed a meta-analysis separately for childhood CVT, including their own study results, and showed a significant association between PTM and childhood CVT (OR, 5.3; 95% CI, 1.4-19.8), but not between FVL and childhood CVT (OR, 2.3; 95% CI, 0.8-6.3).23 The opposite outcome compared with the meta-analysis of Kenet et al is probably caused by the low number of patients included in both meta-analyses.
Value of thrombophilia testing
In general, thrombophilia testing should only be performed if the results would change management. As discussed in the previous paragraphs, thrombophilia contributes to VTE in neonates and children, but its extent depends on the specific patient group. Although thrombophilia testing is often performed to gain insight into the cause of VTE in a child, identification of thrombophilia should not be considered the sole cause. Almost all children have 1 or more clinical risk factors, and unprovoked thrombosis occurs in only 5% of the children.2
Unprovoked thrombosis and a family history of VTE may help to identify thrombophilia in children with VTE. Revel-Vilk et al studied thrombophilia in 171 children with VTE. Thrombophilic disorders were present in no more than 13% of the total number of patients, but in 60% of the adolescents with unprovoked thrombosis.30 In a prospective cohort study of 100 children with VTE, family history of VTE appeared to be the only predictor for the presence of inherited thrombophilia (OR, 14.9; 95% CI, 1.9-113) after multivariate analysis.31 Likewise, in the cross-sectional study by Ruud et al, family history of VTE increased the RR of a child having inherited thrombophilia to 2.35 (95% CI, 1.1-5.2).32 However, associations are not strong, and in adults, family history has a poor predictive value for the presence of inherited thrombophilia.33
In children with thrombosis, the most important justification for thrombophilia testing would be to distinguish patients at high risk of recurrent VTE from those at low risk. Patients at high risk might benefit from long-term anticoagulation. In adults, guidelines do not recommend thrombophilia testing to guide decisions on duration of anticoagulation, as inherited thrombophilia is considered to play a minor role in recurrence risk, with RR estimates between 1.4 and 2.5.24 In children, the influence of inherited thrombophilia on recurrent thrombosis is very similar. Young et al showed an association between recurrent VTE in children and all inherited thrombophilias, except FVL. The summary ORs ranged from 1.88 (95% CI, 1.01-3.29) for PTM to 4.46 (95% CI, 2.89-6.89) for combined disorders.6 Prospective trials studying the risks and benefits of prolonged anticoagulation treatment in pediatric thrombophilic patients have not been performed. Until now, as in adults, duration of anticoagulant therapy in children is not modified based on the presence of thrombophilia.34
A potential advantage of testing pediatric patients with VTE for inherited thrombophilia is the identification of asymptomatic siblings with thrombophilia. Although the incidences of VTE in carriers of AT, PC, or PS deficiency are higher than in those with FVL or PTM, and clearly higher than in relatives who do not have thrombophilia, testing is not generally recommended (Table 2).35 However, it might be beneficial to identify carriers of high-risk thrombophilia in thrombosis-prone families. Asymptomatic relatives with deficiencies of AT, PC, or PS are thought to benefit from primary prophylaxis with anticoagulants during high-risk situations, and female carriers can be counseled about the use of combined oral contraceptives and thromboprophylaxis during pregnancy and puerperium.24,36 However, in our view, negative thrombophilia testing may provide false reassurance. For example, in high-risk thrombophilia families, the risk of VTE during oral contraceptive use is indeed increased in affected relatives (4.3% per year of use), but also in unaffected relatives (0.7% per year of use) compared with the general population (0.04% per year of use). This is probably caused by the cosegregation of as yet unidentified genetic variables and/or environmental risk factors.37
If testing is considered, it seems rational to postpone testing asymptomatic children until they are old enough to decide for themselves after weighing the pros and cons, as the absolute incidence of VTE is very low in children with thrombophilia.38 Furthermore, diagnosing a true deficiency of AT, PC, and PS can be challenging in young children because of the rapidly developing hemostatic system in the first year of life and the presence of physiological significantly lower levels of certain coagulation inhibiters compared with adults until adolescence.39
Conclusion
VTE in childhood is a multifactorial disease, in which underlying clinical risk factors and inherited thrombophilia contribute to the development of VTE.
The presence of a thrombophilia does not change patients’ treatment strategies, and consequently, testing is not advised routinely in all children with VTE, except for study purposes. However, asymptomatic adult relatives in families with high-risk thrombophilia may benefit from testing by offering them primary thromboprophylaxis in high-risk situations and by counseling the female carriers before the use of oral contraceptives and pregnancy, although it should be realized that negative testing may provide false reassurance. Unprovoked thrombosis and a positive family history may help to predict the presence of thrombophilia in children with VTE. As the incidence of VTE is very low in children with thrombophilia, it seems reasonable to postpone testing in asymptomatic children until they are old enough to decide for themselves in view of the pros and cons. Pre- and posttest counseling should be performed by an experienced hematologist or coagulation specialist.
Authorship
Contribution: I.L.M.K. and C.H.v.O. selected the studies after the systematic research; I.L.M.K. wrote the manuscript; and C.H.v.O. and S.M. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Saskia Middeldorp, Department of Vascular Medicine, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: s.middeldorp@amc.uva.nl.