In this issue of Blood, Bruneau et al1 provide evidence that disruption of diacylglycerol kinase ε isoform (DGKε) does not upregulate complement activation, but rather induces endothelial damage via the activation of p38 mitogen–activated protein kinase (MAPK). These in vitro findings may support a new pathophysiologic mechanism for atypical hemolytic uremic syndrome (aHUS) in a subset of patients with DGKE gene mutations.
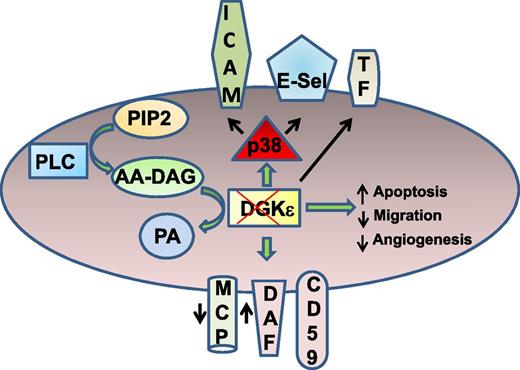
Disrupting DGKε in endothelial cells (ECs) leads to prothrombotic phenotype without complement activation. After receptor activation, phospholipase C (PLC) mediates hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) to DAG. Arachidonic acid–containing DAG is catalyzed to PA by DGKε. Disruption of DGKε increases p38 activation and surface expression of intercellular adhesion molecule-1 (ICAM-1), E-selectin (E-Sel), and tissue factor (TF). Loss of DGKε also increases endothelial apoptosis, impairing migration and angiogenic response. DGKε depletion decreases the surface expression of membrane cofactor protein (MCP), increases the expression of decay accelerating factor (DAF), and does not alter the level of CD59. Despite these changes, complement C3b deposition was not altered in DGKε-deleted cells.
Disrupting DGKε in endothelial cells (ECs) leads to prothrombotic phenotype without complement activation. After receptor activation, phospholipase C (PLC) mediates hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) to DAG. Arachidonic acid–containing DAG is catalyzed to PA by DGKε. Disruption of DGKε increases p38 activation and surface expression of intercellular adhesion molecule-1 (ICAM-1), E-selectin (E-Sel), and tissue factor (TF). Loss of DGKε also increases endothelial apoptosis, impairing migration and angiogenic response. DGKε depletion decreases the surface expression of membrane cofactor protein (MCP), increases the expression of decay accelerating factor (DAF), and does not alter the level of CD59. Despite these changes, complement C3b deposition was not altered in DGKε-deleted cells.
aHUS is a thrombotic microangiopathy characterized by thrombocytopenia, anemia, and microthrombi found predominantly in the kidney. Recurrent clinical course is common, and end-stage renal disease develops in more than half of the affected individuals. Mutation in one or more of the genes that encode proteins of the alternative complement pathway, such as factor H, factor I, membrane cofactor protein (MCP), complement factor H–related proteins CFHR1 and CFHR3, thrombomodulin, and factors B and C3, is a hallmark of this disease. Mechanistically, these mutations render excessive complement activation, which damages glomerular endothelial cells and likely supports microthombi via local tissue factor exposure, thrombin generation, and platelet adhesion/aggregation. Indeed, a monoclonal antibody to complement C5 (eculizumab) has proven efficacious in aHUS patients with complement defect.
The strong complement-aHUS link was challenged when 2 independent reports in 2013 revealed recessive loss-of-function mutations in the DGKE gene in a subset of patients with aHUS and membranoproliferative glomerulonephritis, respectively.2,3 DGKE encodes for an enzyme DGKε that is distinct from the complement pathway. In fact, DGKε is a lipid kinase that can phosphorylate specifically diacylglycerol (DAG) with an arachidonoyl group at the sn-2 position of the glycerol backbone and generate phosphatidic acid (PA) (see figure).4 DAG is generated predominantly by PLC–mediated hydrolysis of PIP2 downstream of G protein–coupled receptors and integrins. Thus, loss-of-function mutations in DGKE are predicted to alter the intracellular levels of arachidonic acid containing DAG and PA, with potential changes in cellular signaling downstream of these bioactive lipids. How disruption of DGKE might contribute to the pathophysiology of aHUS is currently unknown.
The article by Bruneau et al1 is timely and begins to address this important and significant question. The authors show that disruption of DGKE by siRNA from ECs of 2 different vascular beds (human umbilical vein ECs and human microvascular ECs) can enhance expression of ICAM-1, E-Sel, and TF, with a concomitant increase in platelet adhesion (see figure). DGKε-depleted ECs revealed an increase in p38α MAPK signaling in phosphoprofiling studies. More importantly, p38 inhibitor blocked the increased ICAM-1 and E-Sel expression in DGKε-depleted ECs. Thus, loss of DGKε can trigger endothelial activation and display a prothrombotic phenotype. Intriguingly, the authors also show that disruption of DGKE induces EC apoptosis, impairing migration (wound healing assays) and angiogenic response (tube formation assays). The findings suggest that loss of DGKε likely promotes vascular damage. Finally, knockdown of DGKE caused a differential effect on the surface expression of complement inhibitory proteins. For example, the expression of MCP was decreased, whereas DAF expression was increased and the level of membrane attack complex–inhibitory protein (MAC-IP; CD59) remained unchanged. Importantly, these changes in the complement regulatory proteins did not increase C3b deposition on DGKε-depleted ECs, suggesting that complement activation is unlikely to be the trigger for endothelial damage.
This study has implications for both basic and clinical science associated with DGKε. From the perspective of basic science, this study provides some unexpected biological roles for endothelial DGKε. In a simplistic view of signal transduction, DGKε is thought to attenuate signaling initiated by arachidonic acid containing DAG and/or promote signaling mediated by PA. Therefore, loss of DGKε is expected to trigger enhanced signaling via downstream effectors of DAG, including protein kinase C.5 Yet, the authors noticed robust phosphorylation of p38 Thr180 and Tyr182, and only a modest increase in the phosphorylation of PKC Ser660, surrogate markers of p38 and PKC activation. Functionally, DGKε-depleted ECs showed signs of endothelial activation (increase in ICAM-1, TF expression) without changes in P-selectin or von Willebrand secretion. These perplexing findings in DGKε-depleted ECs highlight the complexity of DGKε signaling and likely reflect a crosstalk between both DAG- and PA-mediated signaling. Their data also raise additional questions, such as: (1) How does loss of DGKε activate p38? (2) How can loss of DGKε facilitate apoptosis, impair migration, and support endothelial damage? (3) Platelets also express DGKε; could the loss of DGKε affect platelet function? From the clinical point of view, this study provides some insights into the pathophysiologic mechanisms that may underpin aHUS in a cohort with DGKE mutations. The data suggest that endothelial activation and damage independent of complement activation may contribute to the disease and thus challenge the benefit of complement blockade under these conditions. Consistent with Bruneau et al's interpretation, at least 2 aHUS patients with a DGKE mutation had relapse of disease while on therapy with eculizumab and infusion of fresh frozen plasma.2
The challenge now lies in verifying whether the increased p38 activation identified by the authors can be translated beyond the in vitro ECs and into glomerular podocytes and ECs obtained from kidney biopsy of the affected individuals. Finally, if p38 activation is validated in patients with DGKE mutations, could p38 inhibitors, which are being tested in phase 2 clinical trials for inflammatory disorders like rheumatoid arthritis,6 have some therapeutic value in a subset of eculizumab-nonresponsive aHUS patients with DGKE mutations?
Conflict-of-interest disclosure: The author declares no competing financial interests.