Key Points
G-CSF suppresses B lymphopoiesis at multiple stages of development.
G-CSF reprograms bone marrow stromal cells to inhibit their production of B-cell trophic factors.
Abstract
The mechanisms that mediate the shift from lymphopoiesis to myelopoiesis in response to infectious stress are largely unknown. We show that treatment with granulocyte colony-stimulating factor (G-CSF), which is often induced during infection, results in marked suppression of B lymphopoiesis at multiple stages of B-cell development. Mesenchymal-lineage stromal cells in the bone marrow, including CXCL12-abundant reticular (CAR) cells and osteoblasts, constitutively support B lymphopoiesis through the production of multiple B trophic factors. G-CSF acting through a monocytic cell intermediate reprograms these stromal cells, altering their capacity to support B lymphopoiesis. G-CSF treatment is associated with an expansion of CAR cells and a shift toward osteogenic lineage commitment. It markedly suppresses the production of multiple B-cell trophic factors by CAR cells and osteoblasts, including CXCL12, kit ligand, interleukin-6, interleukin-7, and insulin-like growth factor-1. Targeting bone marrow stromal cells is one mechanism by which inflammatory cytokines such as G-CSF actively suppress lymphopoiesis.
Introduction
Under basal conditions, bone marrow stromal cells provide signals that support a balance between lymphopoiesis and myelopoiesis. In response to infectious stress, there is a shift in hematopoiesis in the bone marrow from lymphopoiesis to granulopoiesis. This is mediated, at least in part, by granulocyte colony-stimulating factor (G-CSF), which is often induced during the acute phase of bacterial infection,1,2 and it is known to suppress lymphopoiesis while stimulating granulopoiesis.3,4 A recent study showed that G-CSF suppresses B lymphopoiesis in the bone marrow at multiple stages of development, in part by inducing apoptosis of B-cell precursors.5 B lymphopoiesis is dependent on the production of supportive signals by bone marrow stromal cells, including CXCL12-abundant reticular (CAR) cells, osteoblasts, and other stromal cells.6-8 Whether G-CSF affects the capacity of these stromal cells to support B lymphopoiesis is unknown.
Study design
Mice
Transplantation
Bone marrow from Csf3r−/− mice expressing Ly5.2 was mixed at a 2:1 ratio with wild-type marrow expressing Ly5.1/5.2 and transplanted retro-orbitally into irradiated Ly5.1 recipients as previously described.10
Flow cytometry and sorting
To extract stromal cells, femurs and tibias were crushed in phosphate-buffered saline. Cells in suspension were collected and stored on ice while bone chips were digested by using collagenase type II (3 mg/mL) and dispase (4 mg/mL) at 37°C for 1 hour. Cells were then processed for flow cytometry as described previously.10 A list of antibodies used is provided in supplemental Table 2, available on the Blood Web site. Cells were analyzed by using a Gallios flow cytometer. Cell sorting was performed on a Synergy cytometer.
Immunostaining
Femurs and tibias were fixed for 16 to 24 hours in 4% paraformaldehyde at 4°C. Bones were decalcified in 14% EDTA (pH 7.4) solution for 3 to 5 days and cryoprotected in 30% sucrose for 16 to 24 hours. Bones were then snap frozen in optimal cutting temperature media, and tissue blocks were sectioned using the CryoJane tape-transfer system. Slides were imaged by using an LSM 700 confocal microscope and ZEN imaging software. Velocity image processing software was used to calculate distances between cells.
RNA expression profiling
RNA from sorted CAR cells was amplified by using the NuGen Ovation system and hybridized to the Affymetrix MoGene 1.0 ST array. Data were normalized by using the robust multichip average algorithm. The RNA expression data are available through Gene Expression Omnibus (GSE67104).
Statistics
Statistical significance of differences was calculated for 2 groups by using the Student t test and for 3 or more groups by using 1- or 2-way analysis of variance. All data are presented as mean ± standard error of the mean. The RNA expression profiling data were analyzed by using statistical analysis of microarrays.
Results and discussion
Consistent with a previous report,5 we observed that G-CSF treatment resulted in marked loss of B cells in the bone marrow, which reached its nadir at 7 days and recovered to near normal 7 days after stopping G-CSF (Figure 1A). To determine whether G-CSF acts in a cell-intrinsic fashion to suppress B lymphopoiesis, we generated Csf3r−/− mixed bone marrow chimeras by transplanting a mixture of wild-type and Csf3r−/− bone marrow into wild-type recipients. Following G-CSF treatment, wild-type B cells in the bone marrow of the chimeras were reduced 10-fold compared with those in untreated mice (Figure 1B). Importantly, a similar decrease in Csf3r−/− B cells was observed (12-fold decrease). In contrast, G-CSF is known to act in a cell intrinsic fashion to stimulate granulopoiesis.11 Accordingly, G-CSF treatment of the mixed chimeras resulted in an expansion of only Csf3r+/+ neutrophils (Figure 1C).
G-CSF works through cells of the monocyte-macrophage lineage to suppress B lymphopoiesis. (A) Wild-type mice were treated with G-CSF (250 µg/kg per day) or saline alone for the indicated time, and the B cells in the bone marrow were quantified; 7P, 7 days after stopping G-CSF (n = 3-12). (B-C) Mixed chimera mice were generated by transplanting wild-type (Ly5.1/Ly5.2) and Csf3r−/− (Ly5.2) bone marrow cells into irradiated wild-type (Ly5.1) recipients at a 1:2 ratio. Eight weeks after transplantation, mice were treated with G-CSF for 5 days or left untreated, and (B) B cells and (C) neutrophils were quantified by flow cytometry (n = 4-5). (D) CD68:Csf3r, Csf3r−/− mice were treated for 5 days with phosphate-buffered saline (PBS) or G-CSF. Shown is the number of B cells in the bone marrow for each B-cell subset (n = 7-8). (E) CD68:Csf3r, Csf3r−/− femurs were flushed with Trizol to collect total bone marrow RNA. Shown is the messenger RNA (mRNA) expression of the indicated gene relative to β-actin mRNA (n = 7-8). *P < .05; ***P < .001. ns, not significant.
G-CSF works through cells of the monocyte-macrophage lineage to suppress B lymphopoiesis. (A) Wild-type mice were treated with G-CSF (250 µg/kg per day) or saline alone for the indicated time, and the B cells in the bone marrow were quantified; 7P, 7 days after stopping G-CSF (n = 3-12). (B-C) Mixed chimera mice were generated by transplanting wild-type (Ly5.1/Ly5.2) and Csf3r−/− (Ly5.2) bone marrow cells into irradiated wild-type (Ly5.1) recipients at a 1:2 ratio. Eight weeks after transplantation, mice were treated with G-CSF for 5 days or left untreated, and (B) B cells and (C) neutrophils were quantified by flow cytometry (n = 4-5). (D) CD68:Csf3r, Csf3r−/− mice were treated for 5 days with phosphate-buffered saline (PBS) or G-CSF. Shown is the number of B cells in the bone marrow for each B-cell subset (n = 7-8). (E) CD68:Csf3r, Csf3r−/− femurs were flushed with Trizol to collect total bone marrow RNA. Shown is the messenger RNA (mRNA) expression of the indicated gene relative to β-actin mRNA (n = 7-8). *P < .05; ***P < .001. ns, not significant.
G-CSF works through cells of the monocyte-macrophage lineage to mobilize hematopoietic progenitor cells from the bone marrow.12-14 To determine whether monocyte-macrophage lineage cells are also responsible for mediating G-CSF–induced B-cell suppression, we used CD68:Csf3r, Csf3r−/− transgenic mice in which the G-CSF receptor was expressed only on monocyte-macrophage lineage cells.12 After 5 days of G-CSF treatment, bone marrow B-cell number was significantly decreased (Figure 1D). To determine which stages of B-cell development were affected, we measured B-cell progenitor populations in the bone marrow (Figure 1D). Consistent with a prior study of wild type mice,5 G-CSF treatment of CD68:Csf3r, Csf3r−/− transgenic mice resulted in loss of B-cell precursors. Together, these data show that G-CSF acts through a monocytic-cell intermediate to suppress B lymphopoiesis at multiple stages of development. The factor(s) produced by monocytic cells upon G-CSF stimulation that suppress B lymphopoiesis are currently unknown.
We next examined the bone marrow microenvironment for potential candidates mediating the G-CSF–induced B-cell suppression. Bone marrow stromal cells are known to produce several trophic factors important for B-cell development. We observed significant decreases in CXCL12, interleukin-6 (IL-6), IL-7, kit ligand, FLT3L, insulin-like growth factor-1 (IGF-1), and B-cell–activating factor (BAFF) messenger RNA (mRNA) in the bone marrow (Figure 1E). Likewise, protein expression of CXCL12 and BAFF was significantly reduced in the bone marrow after G-CSF treatment (supplemental Figure 1).
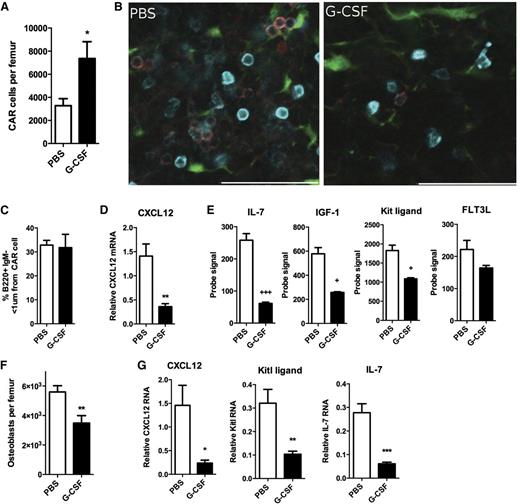
CAR cells are a major source of CXCL12 and other B-cell trophic factors in the bone marrow.15,16 We used transgenic mice carrying a knockin of the green fluorescent protein gene (Gfp) into the Cxcl12 locus to assess CAR cells.7 The number of CAR cells was modestly increased after G-CSF treatment (Figure 2A and supplemental Figure 2). Consistent with prior reports, we show that B-cell precursors in the bone marrow localize near CAR cells (Figure 2B).7 After G-CSF treatment, although the number of B-cell precursors was markedly reduced, the remaining cells remained in close proximity to CAR cells (Figure 2C). RNA expression profiling of sorted CAR cells after G-CSF treatment revealed that CAR cells constitutively express CXCL12, IL-7, Kit ligand, IGF-1, and Flt3 ligand, and expression of all of these genes, except Flt3 ligand, is decreased following G-CSF treatment (Figure 2D and supplemental Table 1). Interestingly, G-CSF treatment appears to enhance the osteogenic potential of CAR cells while suppressing adipogenesis. Specifically, G-CSF suppressed expression of genes associated with adipocyte differentiation in CAR cells and increased the number of osteoblastic colony-forming cells (supplemental Figure 3).
G-CSF targets expression of B-trophic factors in CAR cells and osteoblasts. (A) Cxcl12gfp mice were treated for 7 days with G-CSF or PBS, and CAR cells in the bone marrow were quantified by flow cytometry. (B) Representative photomicrographs showing the relationship of CAR cells to B220+ (cyan) immunoglobulin M (IgM–) (red) B-cell precursors. Scale bar = 50 μm. (C) Quantification of the number of B220+IgM– cells within 1 µm of a CAR cell (n = 7-11). (D) Expression of CXCL12 mRNA relative to β-actin mRNA in sorted CAR cells (n = 4-5). (E) RNA expression profiling of sorted CAR cells was performed. Shown are probe signals for the indicated genes (n = 4-5). (F) Col2.3-Gfp mice were treated for 5 days with G-CSF or PBS, and the number of Col2.3-GFP+lineage–CD45– osteolineage cells was quantified by flow cytometry (n = 7). (G) The expression of the indicated genes relative to β-actin in sorted osteoblasts is shown (n = 7). Statistics by two-tailed Student t test, *P < .05; **P < .01; ***P < .001; or statistical analysis of microarrays, +FDR < 0.05; +++FDR < 0.001. FDR, false discovery rate.
G-CSF targets expression of B-trophic factors in CAR cells and osteoblasts. (A) Cxcl12gfp mice were treated for 7 days with G-CSF or PBS, and CAR cells in the bone marrow were quantified by flow cytometry. (B) Representative photomicrographs showing the relationship of CAR cells to B220+ (cyan) immunoglobulin M (IgM–) (red) B-cell precursors. Scale bar = 50 μm. (C) Quantification of the number of B220+IgM– cells within 1 µm of a CAR cell (n = 7-11). (D) Expression of CXCL12 mRNA relative to β-actin mRNA in sorted CAR cells (n = 4-5). (E) RNA expression profiling of sorted CAR cells was performed. Shown are probe signals for the indicated genes (n = 4-5). (F) Col2.3-Gfp mice were treated for 5 days with G-CSF or PBS, and the number of Col2.3-GFP+lineage–CD45– osteolineage cells was quantified by flow cytometry (n = 7). (G) The expression of the indicated genes relative to β-actin in sorted osteoblasts is shown (n = 7). Statistics by two-tailed Student t test, *P < .05; **P < .01; ***P < .001; or statistical analysis of microarrays, +FDR < 0.05; +++FDR < 0.001. FDR, false discovery rate.
Osteoblasts are an important component of the bone marrow lymphoid niche.8,15 As reported previously,17-19 we show that G-CSF treatment results in a loss of osteoblast lineage cells in the bone marrow (Figure 2F). Similar to CAR cells, G-CSF markedly suppressed the expression of several key B-cell trophic factors, including CXCL12, IL-7, and kit ligand in sorted Col2.3-GFP+ stromal cells (Figure 2G). A previous study showed that G-CSF also targets Nestin-GFP+ stromal cells, altering their expression of certain hematopoietic stem cell maintenance genes.13 Thus, G-CSF treatment appears to broadly affect stromal cells that compose the lymphoid and stem cell niches.
Our data show that G-CSF actively suppresses lymphopoiesis by targeting stromal cells that contribute to lymphoid niches in the bone marrow. Because it is often induced in response to infectious stress, G-CSF provides a mechanism to broadly shape hematopoiesis through regulation of the bone marrow microenvironment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr David Rowe for the Col2.3gfp mice.
This work was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grants RO1 HL60772 (D.C.L.) and F30 HL112552-02 (R.B.D.) and NIH National Cancer Institute grant P50 CA171963 (D.C.L.). D.B. is a New York Stem Cell Foundation Robertson Investigator.
Authorship
Contribution: R.B.D., D.B., and D.C.L. designed and wrote the manuscript; R.B.D. performed the experiments; and T.N. provided the Cxcl12gfp mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel C. Link, Division of Oncology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: dlink@dom.wustl.edu.