Key Points
Rac deletion in Nestin+ cells reverses the arteriolar-to-sinusoid ratio in marrow.
Rac-deleted Nestin+ cells differentially alter long-term HSC and hematopoietic progenitors.
Abstract
Hematopoietic stem cells (HSCs) are localized within specialized microenvironments throughout the BM. Nestin-expressing (Nestin+) mesenchymal stromal cells (MSCs) are important in the perivascular space. Rac is critical for MSC cell shape in vitro, whereas its function in MSCs in vivo remains poorly characterized. We hypothesized that deletion of Rac in the Nestin+ cells would perturb the perivascular space, altering HSC localization and hematopoiesis. Nestin-Cre–directed excision of Rac1 in Rac3−/− mice reduces Nestin+ cells in the marrow. We observed a 2.7-fold decrease in homing of labeled wild-type hematopoietic cells into Rac1Δ/ΔRac3−/− mice compared with control mice. Rac1Δ/ΔRac3−/− mice demonstrated a marked decrease in arterioles and an increase in the number and volume of venous sinusoids in the marrow that was associated with a reduction in the numbers of immunophenotypically and functionally-defined long-term HSCs in the marrow, a decrease in colony-forming cells and a reduction in circulating progenitors. Rac-deleted animals demonstrated a significant increase in trabecular bone. These data demonstrate that Rac GTPases play an important role in the integrity of perivascular space. Increased trabecular bone and sinusoidal space and decreased arteriolar volume in this model were associated with decreased HSC, underscoring the complexity of regulation of hematopoiesis in the perivascular space.
Introduction
Hematopoietic stem cells (HSCs) reside in the bone marrow (BM) cavity, a highly-organized and complex microenvironment that supports hematopoiesis and is commonly referred to as the “HSC niche.”1 Cellular and extracellular components of the niche have been implicated in localization of HSCs and HSC self-renewal and differentiation.2-6 Several previous studies have identified key cellular components of the niche, including progeny of mesenchymal stem cells such as osteoblasts, adipocytes, perivascular cells, and endothelial cells.4,7-9 As improved profiling of cell surface markers and more sophisticated isolation techniques have become available, knowledge of specific cell types within the hematopoietic microenvironment (HM) and their functions has advanced, but whether multiple HSC niches that serve distinct functions are present in the marrow remains controversial. Key factors involved in regulation of HSCs by the HM include Stem cell factor (Scf) and C-X-C motif chemokine 12 (Cxcl12, also known as stromal cell–derived factor 1) (reviewed in Anthony and Link10 ). Perivascular mesenchymal stromal cells and endothelial cells express both membrane-bound and soluble forms of Scf, which promotes HSC maintenance in the HM.11 Cxcl12 is mainly produced by CXCL12-abundant reticular (CAR) cells, as well as other stromal cells, and has been shown to be critical for the retention and maintenance of HSCs in the BM.9,12-14
Genetic manipulation of discrete cellular components of the HM often results in impaired HSC numbers but can also affect the function of other cell types within the HM (reviewed in Joseph et al15 ). These studies have underscored the complexity of cellular interactions required to support hematopoiesis and contribute to the evolution of competing models attributing importance to various HSC niches. MSCs are progenitors of multiple cell types in the HM and produce multiple factors important in the maintenance of HSCs.9,12,14 Recent studies have focused on a specific subset of MSCs with predominant perivascular distribution. These cells are present in both humans and mice, colocalize with HSCs and marrow vessels, are believed to be the main component of the vascular HSC niche, and are characterized by expression of the intermediate filament protein, nestin (Nestin+ cells).4,16 Within the vascular niche, the venous sinusoidal compartment represents the most abundant blood vessel type in the BM, and both perivascular MSCs and HSCs colocalize to venous sinusoids.9,17,18 Two groups have reported that the marrow arteriolar vessel microenvironment may be critical to the maintenance of quiescent HSCs.19,20
Bone marrow–derived Nestin+ cells have been shown to retain adipogenic, osteogenic, and chondrogenic capacity in vitro and can give rise to stromal progenitors and early endothelial and osteoblast cells in vivo.4,20,21 More recent studies using a Nestin–green fluorescent protein (GFP) reporter transgenic line have shown that cells expressing high levels of GFP are mainly found around arterioles, whereas low GFP-expressing cells colocalize with marrow sinusoids.19 Several groups have reported that various Nestin transgenes are associated with different expression patterns within the HM9,15,20 (reviewed in Joseph et al15 ) and have demonstrated an overlap between Nestin-GFP+ perivascular cells and Leptin Receptor–expressing (LepR+) MSC cells.9,21 Recent work has suggested that LepR+ cells are critical for the maintenance of quiescent HSCs18 and represent the major source of factors including Scf and Cxcl12 that regulate HSCs in the BM.9 Deletion of Scf in the LepR+ cells has been shown to be associated with depletion of quiescent HSCs from the BM,9,22 whereas excision of Cxcl12 in LepR+ cells induces HSC mobilization.23 In contrast, Nestin-Cre–mediated deletion of Scf and Cxcl12 has not been associated with a significant HSC phenotype.23 These differences in importance of various cell types in the HM in supporting HSCs in lineage-specific knockout mouse models may in part reflect functional redundancies.
Rho GTPases are members of the Ras superfamily that regulate cytoskeletal functions and signal transduction pathways in mammalian cells.24 Of these, the highly homologous Rac1, the hematopoietic-specific Rac2, and Rac3 GTPases have been the best studied in hematopoietic cells. Rac protein function in HSCs to regulate homing, marrow retention, and engraftment25,26 (reviewed in Troeger and Williams27 ). In addition, in vitro data suggest that Rac signaling is important for fibroblast survival28 and osteoblast migration, adhesion, proliferation, and spreading.29,30 Rac1 deletion in a preosteoblastic cell line inhibits differentiation into chondrocytes.31 Previous work from our laboratory has demonstrated that lineage-specific deletion of Rac genes in osteoblastic precursor cells in vivo leads to a significant decrease in trabecular bone formation and abnormal bone architecture, whereas hematopoiesis remains unaffected.2 Thus, Rac signaling appears important in mesenchymal stem/stromal cells in addition to hematopoietic cells.
To understand the role of Rac signaling within the perivascular niche of HM, we deleted Rac1 and Rac3 genes in Nestin+ cells. Here we show a marked increase and morphologic alteration in marrow venous sinusoid spaces accompanied by a decrease in arteriolar structures, reduced homing of wild-type (WT) low-density bone marrow (LDBM) cells into the marrow of transgenic (TG) Rac1Δ/ΔRac3−/− mice, and a significant increase in trabecular bone. These alterations were accompanied by reduction in immunophenotypically and functionally defined long-term repopulating cells and myeloid progenitor cells associated with decreased expression of HSC-supporting chemokines and cytokines in the marrow space.
Material and methods
Isolation of Nestin+ cells
Nestin+ cells were isolated as previously described.4,21 In brief, BM cells from femora, tibia, and sacral bones were gently flushed in L-15 fluorescence-activated cell sorting (FACS) buffer (Invitrogen, Carlsbad, CA). The plugs were digested in 1 mg/mL collagenase type IV (Sigma-Aldrich, Grand Island, NJ) dissolved in Hank’s Balanced Salt Solution (HBSS, Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Thermo-Scientific, Utah) for 30 minutes at 37°C. The supernatant was collected and kept on ice in sorting buffer. The stromal cells contained in the CD45-depleted fraction of BM cells were enriched by immunomagnetic depletion using anti-CD45 magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. The CD45-depleted cell fraction was incubated with directly conjugated antibodies against CD45 (clone 30-F11), CD31 (clone 390), Ter119 (clone TER-119), CD140a (PDGFRα, clone APA5), and CD51 (clone RMV-7), all from BD Bioscience (San Jose, CA). We defined Nestin+ cells as the population of CD140a (PDGFRα) and CD51 double-positive, CD45–CD31–Ter119– cells. Using FACSAria (BD Bioscience) the live CD140a+CD51+CD45–CD31–Ter119– cells were isolated. Postsort purity was >95%.
Immunofluorescence microscopy of BM sections
Femurs of killed mice from each genotype were isolated, fixed in paraformaldehyde-lysine-periodate buffer, and snap frozen in O.C.T. (Tissue-Tek, Sakura, Torrance, CA) as described in Nombela-Arieta et al.32 Five-micron longitudinal femoral cryosections were obtained using the CryoJane tape transfer system (Leica Microsystems). Slides were incubated with antibodies against endoglin (polyclonal), laminin (polyclonal), and Sca-1 (clone E13-161.7), followed by fluorescent dye-conjugated secondary antibodies with 4′,6-diamidino-2-phenylindole (DAPI), and further analyzed by laser scanning cytometry (LSC; Thorlabs, Newton, NJ). Whole-mount immunostaining of femoral BM slices were prepared as described in Nombela-Arieta et al.32 Whole-mount femoral slices were stained with rabbit anti-laminin (polyclonal) and rat anti-Sca1 (clone E13-161.7), goat anti-endoglin (polyclonal), followed by DyLight488-labeled donkey anti-rabbit IgG, DyLight549-labeled donkey anti-rat IgG, and DyLight649-labeled donkey anti-goat IgG (all from Jackson ImmunoResearch, West Grove, PA). Fluorescent photomicrographs were recorded using a confocal/multiphoton scanning microscope controlled by Laser-Sharp software (Bio-Rad Laboratories, Waltham, MA). Confocal microscopy was performed with a Zeiss LSM510/710 system. Image stacks were processed and rendered into 3-dimensional (3D) volumes using Volocity Software (PerkinElmer, Waltham, MA).
Results
Rac deletion in the perivascular space reduces the number of Nestin+ cells and impairs homing of LDBM cells to the marrow
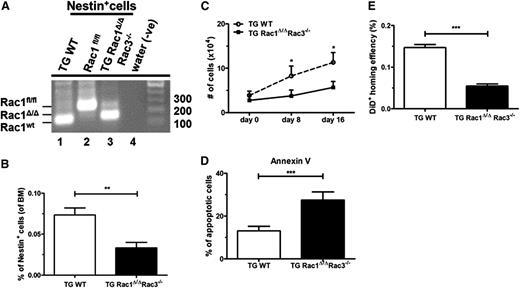
Nestin+ cells are thought to be an important constituent of the perivascular niche in the BM microenvironment, secreting several growth and survival factors.21,33 Previous work from our laboratory has shown that Rac proteins are essential for survival and proliferation of MSCs.2 To understand the role of Rac GTPases in the perivascular niche, Rac1 was conditionally excised in vivo in Nestin+ cells using the Nestin-Cre transgenic mouse mated to Rac1fl/fl mice in the Rac3null genetic background.34 Because the expression of Rac2 is limited to hematopoietic cells, the resulting TG Rac1Δ/ΔRac3−/− cross represents functional deletion of all Rac GTPases in the perivascular space. Nestin+ cells have been shown to be highly enriched in CD45–, CD31–, Ter119–, PDGFRα+, and CD51+ stromal cells.21 Following the gating strategy shown in supplemental Figure 1A (available on the Blood Web site), we isolated Nestin+ cells from TG Rac1Δ/ΔRac3−/− and control mice. To validate the deletion of Rac gene sequences in the Nestin+ cells, live CD45/CD31/Ter119– and PDGFRα+CD51+ cells were purified by FACS sorting from 8- to 14-week-old animals. Postsort purity was shown by flow analysis to be 97%. Genomic (g) DNA from the sorted Nestin+ cells from TG Rac1Δ/ΔRac3−/− and TG WT (Nestin-Cre Rac1WT Rac3WT) mice demonstrated significant, although not complete, deletion of Rac1 sequences in cells isolated from TG Rac1Δ/ΔRac3−/− (Figure 1A). As expected, no deletion of Rac1 sequences was detected in the Nestin+ cells isolated from TG WT mice (Figure 1A). We also documented a modest deletion of Rac1 in CD31+ Nestin– endothelial cell populations (supplemental Figure 1B-C) and in osteoprogenitor cells (supplemental Figure 1D-E). Conditional deletion of Rac1 sequences in the Rac3-null background leads to a significant 2.7-fold decrease in the number of Nestin+ cells in the BM compared with WT mice (Figure 1B). Freshly-sorted Nestin+ cells were seeded and cultured for 16 days in MSC-based culture conditions. Cells derived from TG Rac1Δ/ΔRac3−/− mice showed a significantly impaired twofold reduction in growth in vitro when compared with cells isolated from TG WT animals (Figure 1C). Morphologically, Nestin+ cells from TG Rac1Δ/ΔRac3−/− mice showed a pronounced spindle shape compared with cells derived from WT mice (supplemental Figure 1F), which was previously seen when Rac1 was deleted from the osteoblastic OP9 cell line.2 To determine the mechanism of impaired in vitro growth of Rac1Δ/ΔRac3−/− Nestin+ cells, we analyzed the level of apoptosis of the cultured cells. Nestin+ cells cultured from TG Rac1Δ/ΔRac3−/− showed a twofold increase in apoptotic cells as determined by Annexin V staining when compared with cells isolated from the control mice (Figure 1D). In summary, deletion of Rac1 and Rac3 in perivascular space is associated with decreased numbers of Nestin+ cells in vivo and reduced growth, with increased apoptosis of these isolated cells in vitro.
Rac excision from perivascular niche leads to enhanced apoptosis and decreased numbers of Nestin+ cells and reduced homing of WT cells into the BM of perivascular Rac-deleted mice. (A) Confirmation of deletion of Rac1 sequences in Nestin+ cells isolated by flow cytometry from TG Rac1 Δ/ΔRac3−/− mice. The DNA size ladder is at the right. The position of floxed, deleted (Δ), and WT Rac1 bands are shown at the left. Genotypes are shown above. (B) Percentage of Nestin+ cells defined by expression of CD51+PDGFRα+ in the BM of the genotypes shown below each lane. Data represent mean ± standard deviation (SD); N = 5 experiments repeated four times, **P < .01. (C) Growth of cultured Nestin+ cells from TG WT and TG Rac1Δ/ΔRac3−/− mice. CD51+PDGFRα+ Nestin+ cells were sorted by flow cytometry from BM nucleated cells gated on live CD45/CD31/Ter119– cells (as described in supplemental Figure 1A) and were cultured in vitro over 16 days. Data represent mean ± SD; N = 5 experiments repeated 3 times for each condition, *P < .05 (day 8) and *P < .02 (day 16). (D) Apoptosis in Nestin+ cells isolated from TG WT and TG Rac1Δ/ΔRac3−/− mice as determined by Annexin V+ staining using flow cytometric analysis. Data represent mean ± SD; N = 5 experiments repeated four times, ***P < .001. (E) Homing of DiD-labeled WT LDMB cells injected into recipient mice of each genotype and measured by flow cytometry 12 hours after infusion as described in Material and methods. Data are expressed as % of WT and represent analysis of the equivalent number of live cells/group and time point; mean ± SD, ***P < .001, N = 16 recipients (homing) and experiments were repeated 3 times.
Rac excision from perivascular niche leads to enhanced apoptosis and decreased numbers of Nestin+ cells and reduced homing of WT cells into the BM of perivascular Rac-deleted mice. (A) Confirmation of deletion of Rac1 sequences in Nestin+ cells isolated by flow cytometry from TG Rac1 Δ/ΔRac3−/− mice. The DNA size ladder is at the right. The position of floxed, deleted (Δ), and WT Rac1 bands are shown at the left. Genotypes are shown above. (B) Percentage of Nestin+ cells defined by expression of CD51+PDGFRα+ in the BM of the genotypes shown below each lane. Data represent mean ± standard deviation (SD); N = 5 experiments repeated four times, **P < .01. (C) Growth of cultured Nestin+ cells from TG WT and TG Rac1Δ/ΔRac3−/− mice. CD51+PDGFRα+ Nestin+ cells were sorted by flow cytometry from BM nucleated cells gated on live CD45/CD31/Ter119– cells (as described in supplemental Figure 1A) and were cultured in vitro over 16 days. Data represent mean ± SD; N = 5 experiments repeated 3 times for each condition, *P < .05 (day 8) and *P < .02 (day 16). (D) Apoptosis in Nestin+ cells isolated from TG WT and TG Rac1Δ/ΔRac3−/− mice as determined by Annexin V+ staining using flow cytometric analysis. Data represent mean ± SD; N = 5 experiments repeated four times, ***P < .001. (E) Homing of DiD-labeled WT LDMB cells injected into recipient mice of each genotype and measured by flow cytometry 12 hours after infusion as described in Material and methods. Data are expressed as % of WT and represent analysis of the equivalent number of live cells/group and time point; mean ± SD, ***P < .001, N = 16 recipients (homing) and experiments were repeated 3 times.
Previous studies using intravital microscopy have demonstrated that Nestin+ cells are critical for the directed migration of hematopoietic stem and progenitor cells (HSPCs) to the HSC niche.4 We thus hypothesized that reduction in the number of Nestin+ cells in TG Rac1Δ/ΔRac3−/− mice would functionally impair homing of WT hematopoietic cells to the BM. Freshly isolated and Vybrant DiD-labeled LDBMs, which contain both HSC and progenitor populations, were injected into lethally-irradiated Rac-deleted or control mice. Twelve hours posttransplantation, analysis of homed LDBMs to BM revealed a significant decrease in homing into the TG Rac1Δ/ΔRac3−/− marrow compared with controls (Figure 1E). These data demonstrate that deletion of Rac genes in Nestin+ cells in vivo impairs homing of hematopoietic cells to BM microenvironment.
The vascular endothelial niche is regulated by Rac proteins
The BM vascular niche provides a microenvironment for quiescent HSCs and possibly a site for HSCs to obtain entry to the peripheral circulation.35,36 To evaluate the importance of Rac genes in the vascular niche of the BM, we next performed anti-CD31 staining on femora from TG Rac1Δ/ΔRac3−/− and TG WT mice. There was a significant increase in the number of CD31+ vessels within the BM cavity (data not shown). To specifically identify the type of the vessels that contribute to this increase in vasculature, a 3D reconstruction of a whole-mount section stained for laminin (arteries and sinusoids), endoglin (sinusoids), and Sca-1 (arteries) was assessed for individual mice of each genotype (Figure 2A). Analysis of the vascular architecture revealed a significant increase in sinusoidal volume represented by endoglin-containing vasculature (Figure 2B) and a reproducible decrease in arteriolar volume observed by Sca-1–staining of vasculature (Figure 2C) in 8- 12-week-old TG Rac1Δ/ΔRac3−/− compared with WT mice. To further elucidate the vascular compartment, we used laser scanning cytometry (LSC) (Figure 2D). Data collected from LSC was analyzed using iCys software following the gating strategy as detailed in Material and methods32 (supplemental Figure 2A) of the diaphyseal regions (Figure 2D, left 3 panels) based on endoglin+ signals. There is an increase in the number of sinusoids in the diaphysis of TG Rac1Δ/ΔRac3−/− mice vs control animals. Moreover, high-resolution field images from the indicated genotypes revealed an enlargement of the sinusoidal lumen (as observed in supplemental Videos 1 and 2) in the Rac-deleted vs TG WT animals. The diameters of sinusoids were determined by measuring the distance between opposite walls of sinusoidal lumens. To discriminate the nonsinusoidal vessels, we used software based on automatic detection of endoglin+ vascular structures, which creates peripheral contours around the endoglin+ signal events (Figure 2D, right panel, contour niche signals, and supplemental Figure 2B). Statistical analysis on data collected from endoglin-stained structures using iCys software showed a predominant sinusoidal vessel distribution with the diameter mainly between 10 to 20 µm and above (Figure 2E). These data demonstrate that the deletion of Rac genes in the perivascular Nestin+ cells led to a significant increase in sinusoidal area in the endothelial niche area compared with the control group (Figure 2F). In summary, these data suggest that Rac genes play a key role in the organization of the BM vascular structure.
Rac deletion in Nestin+ cells disturbs the vascular morphology in the medullary cavity. (A) Snapshot images of 3D reconstructed whole-mount sections showing BM microvasculature in the endosteal region of the femoral diaphysis from each genotype after staining for laminin (green, arteries and sinusoids), endoglin (red, sinusoids), and Sca-1 (blue, arteries) as detailed in Material and methods. (B) Sinusoidal volume and (C) arteriolar volume quantification of diaphysis from snapshot images of 3D-reconstructed whole-mount sections (as shown in [A]) was determined using Volocity 3D imaging software. Data represent mean ± standard error of the mean (SEM), N = 4 animals per group, *P < .05, 4-5 individual sections analyzed per mouse. (D) Changes in sinusoidal architecture as assessed LSC of whole longitudinal image of femoral cryosection stained with DAPI (nuclei) showing proximal metaphysis (PM), diaphysis (DIA), and distal metaphysis (DM). Representative LSC images (middle panels) of BM stained for endoglin (red), laminin (green), and DAPI (blue) of TG WT (top panel) and TG Rac1 Δ/ΔRac3−/− mice (bottom panel). The middle panel shows high-resolution images of the indicated field. The right panel show images of contoured niche signals of indicated fields. Analysis of endoglin+ signal events as detailed in Material and methods, based on fluorescence intensity (as shown in supplemental Figure 2A-B) and detected by the iCys software (teal marks). (E) Quantitative analysis of the frequency of sinusoidal distribution by diameter of BM vessels of images in (D, middle panels) determined using iCys software, in a blinded manner. Data represent mean ± SEM, N = 3 animals per group, *P < .05, **P < .01. (F) Vascular sinusoidal niche area quantification of diaphysis from photographs in (D, right panel) was assessed using iCys software. Data represent mean ± SEM, N = 3 animals per group, *P < .05.
Rac deletion in Nestin+ cells disturbs the vascular morphology in the medullary cavity. (A) Snapshot images of 3D reconstructed whole-mount sections showing BM microvasculature in the endosteal region of the femoral diaphysis from each genotype after staining for laminin (green, arteries and sinusoids), endoglin (red, sinusoids), and Sca-1 (blue, arteries) as detailed in Material and methods. (B) Sinusoidal volume and (C) arteriolar volume quantification of diaphysis from snapshot images of 3D-reconstructed whole-mount sections (as shown in [A]) was determined using Volocity 3D imaging software. Data represent mean ± standard error of the mean (SEM), N = 4 animals per group, *P < .05, 4-5 individual sections analyzed per mouse. (D) Changes in sinusoidal architecture as assessed LSC of whole longitudinal image of femoral cryosection stained with DAPI (nuclei) showing proximal metaphysis (PM), diaphysis (DIA), and distal metaphysis (DM). Representative LSC images (middle panels) of BM stained for endoglin (red), laminin (green), and DAPI (blue) of TG WT (top panel) and TG Rac1 Δ/ΔRac3−/− mice (bottom panel). The middle panel shows high-resolution images of the indicated field. The right panel show images of contoured niche signals of indicated fields. Analysis of endoglin+ signal events as detailed in Material and methods, based on fluorescence intensity (as shown in supplemental Figure 2A-B) and detected by the iCys software (teal marks). (E) Quantitative analysis of the frequency of sinusoidal distribution by diameter of BM vessels of images in (D, middle panels) determined using iCys software, in a blinded manner. Data represent mean ± SEM, N = 3 animals per group, *P < .05, **P < .01. (F) Vascular sinusoidal niche area quantification of diaphysis from photographs in (D, right panel) was assessed using iCys software. Data represent mean ± SEM, N = 3 animals per group, *P < .05.
Deletion of Rac in Nestin+ cells is associated with increased trabecular bone formation and increased osteoblasts in vivo
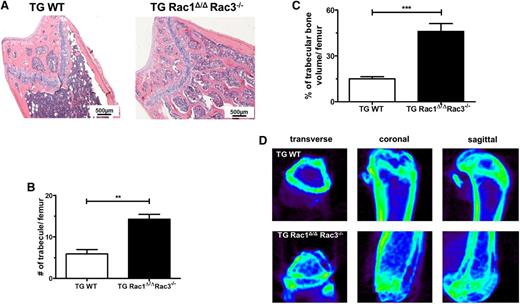
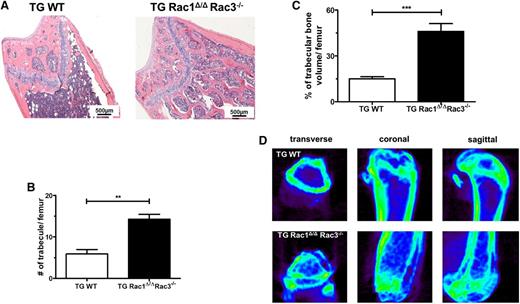
Nestin+ cells are multipotent progenitors that can differentiate into osteoblasts, cartilage, and adipocytes.4,20,37 Previous work from our laboratory has demonstrated that the expression of Rac proteins is required in osteoblasts for normal bone formation in vivo, because the deletion of Rac1 and Rac3 from osteoblasts and their precursors leads to a marked reduction of trabecular bone volume and abnormal bone architecture in mice.2 In hematoxylin and eosin–stained sections of femurs from TG Rac1Δ/ΔRac3−/− and TG WT mice, multiple aberrant medullary bone structures were seen in the trabecular space in TG Rac1Δ/ΔRac3−/− mice (Figure 3A). The number and volume of trabecular bones were determined on sectioned, randomly selected femurs. Lineage-specific deletion of Rac in Nestin+ cells in vivo led to a significant increase in the number of trabeculae (Figure 3B) and an enhanced trabecular bone volume (Figure 3C). Rac-deficient mice also demonstrated increased cortical bone thickness (supplemental Figure 3A). To confirm this data, micro–computed tomography imaging was performed on the femora of TG Rac1Δ/ΔRac3−/− and TG WT mice. Rac-deficient mice showed a marked increase in the trabecular bone formation within the medullary cavity using CT imaging compared with the normal bone architecture seen in TG WT animals (Figure 3D and supplemental Videos 3 and 4).
Deletion of Rac genes in perivascular space leads to abnormal trabecular bone formation in the medullary cavity. Femurs from TG WT and TG Rac1Δ/ΔRac3−/− TG Rac1Δ/ΔRac3−/− mice were prepared and quantification performed as detailed in Material and methods. (A) Hematoxylin and eosin staining of femurs of TG WT and TG Rac1Δ/ΔRac3−/− mice. The scale bar represents 500 μm. Photomicrographs were viewed at 40× original magnification using a Nikon Eclipse 80i microscope, and analyzed with NIS Elements Version 2BR imaging software. (B) Quantification of trabecular bone numbers and (C) trabecular bone volume in medullary cavity space. Data generated using NIS Elements AR software. Data represent mean ± SD, N = 3 animals per group, **P < .01, ***P < .001. (D) Micro-CT images of distal and proximal femoral trabecular bone structure in TG WT and TG Rac1 Δ/ΔRac3−/− mice.
Deletion of Rac genes in perivascular space leads to abnormal trabecular bone formation in the medullary cavity. Femurs from TG WT and TG Rac1Δ/ΔRac3−/− TG Rac1Δ/ΔRac3−/− mice were prepared and quantification performed as detailed in Material and methods. (A) Hematoxylin and eosin staining of femurs of TG WT and TG Rac1Δ/ΔRac3−/− mice. The scale bar represents 500 μm. Photomicrographs were viewed at 40× original magnification using a Nikon Eclipse 80i microscope, and analyzed with NIS Elements Version 2BR imaging software. (B) Quantification of trabecular bone numbers and (C) trabecular bone volume in medullary cavity space. Data generated using NIS Elements AR software. Data represent mean ± SD, N = 3 animals per group, **P < .01, ***P < .001. (D) Micro-CT images of distal and proximal femoral trabecular bone structure in TG WT and TG Rac1 Δ/ΔRac3−/− mice.
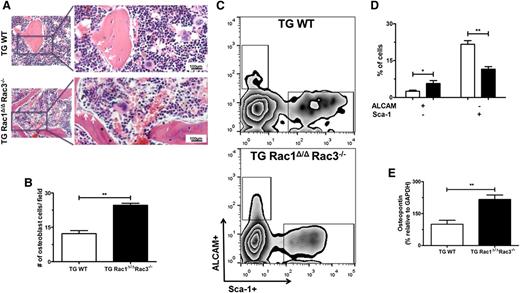
To determine the mechanism of increased trabecular and cortical bone in mice in which Rac genes were deleted in Nestin+ cells, more detailed analysis of femora isolated from TG Rac1Δ/ΔRac3−/− and TG WT mice was performed. Analysis of histologic slides assessed in a blinded fashion demonstrated an increase in the number of osteoblast cells that were aligned along the metaphyseal region and trabecular bone surfaces (Figure 4A, arrows, and Figure 4B) in TG Rac1Δ/ΔRac3−/− mice. As expected, TG WT mice displayed a normal distribution of osteoblasts predominantly on the metaphyseal plate of the bone (Figure 4A-B). To confirm these data, the osteoblast progenitor populations were assessed by flow cytometric analysis. FACS-based analysis of live (7-AAD–) CD45/CD31/Ter119–, ALCAM+Sca1– (osteoblast-enriched population) or ALCAM–Sca1+ (immature MSCs)38,39 (Figure 4C) BM cells demonstrated a significant twofold increase in osteoblasts in TG Rac1Δ/ΔRac3−/− compared with WT mice (Figure 4D). These findings were further supported by an increase in RNA expression levels of the osteoblast markers osteopontin (Opn) (Figure 4E), alkaline phosphatase (ALP) (supplemental Figure 3B), and Runx2 (supplemental Figure 3C) in the freshly isolated Nestin+ cells from TG Rac1Δ/ΔRac3−/− compared with control mice. These findings suggest that Rac deletion in Nestin+ cells leads to increased trabecular and cortical bone formation in vivo by altering the differentiation of these cells into an osteoblast population.
Deletion of Rac genes in Nestin+ cells leads to alterations in osteoblasts populations. (A) Photomicrographs of trabecular bone from mouse femurs after hematoxylin and eosin staining demonstrating osteoblasts (white arrow) recognized as basophilic cells with a cuboidal-columnar shape, arranged in rows along trabecular bone and the inner surface of BM space, and lining osteoblasts presenting a flat shape, lining the bone surface cells (*). The right panel shows expanded views of the boxed area on left. The scale bar represents 100μm; images were viewed at 200× original magnification using a Nikon Eclipse 80i microscope, and analyzed with NIS Elements Version 2BR imaging software. (B) The number of morphologically-defined osteoblasts was quantified from representative animals of each genotype. Data represent mean ± SD, N = 3 animals per group, **P < .01. (C) FACS gating strategy to define osteoblast progenitor cell populations by Sca-1 and ALCAM staining. (D) Percentages of ALCAM and Sca-1–expressing cells in live CD45/CD31/Ter119– BM-associated cell fractions. Data represent mean ± SD, *P < .05, **P < .01, N = 4 individual experiments. (E) Comparison of mRNA levels of HSCs maintenance genes and osteopontin (Opn) from freshly sorted Nestin+ cells from TG WT and TGRac1Δ/Δ/Rac3−/− mice by real-time PCR. Data represent mean ± SD, N = 3 individual experiments, **P < .01.
Deletion of Rac genes in Nestin+ cells leads to alterations in osteoblasts populations. (A) Photomicrographs of trabecular bone from mouse femurs after hematoxylin and eosin staining demonstrating osteoblasts (white arrow) recognized as basophilic cells with a cuboidal-columnar shape, arranged in rows along trabecular bone and the inner surface of BM space, and lining osteoblasts presenting a flat shape, lining the bone surface cells (*). The right panel shows expanded views of the boxed area on left. The scale bar represents 100μm; images were viewed at 200× original magnification using a Nikon Eclipse 80i microscope, and analyzed with NIS Elements Version 2BR imaging software. (B) The number of morphologically-defined osteoblasts was quantified from representative animals of each genotype. Data represent mean ± SD, N = 3 animals per group, **P < .01. (C) FACS gating strategy to define osteoblast progenitor cell populations by Sca-1 and ALCAM staining. (D) Percentages of ALCAM and Sca-1–expressing cells in live CD45/CD31/Ter119– BM-associated cell fractions. Data represent mean ± SD, *P < .05, **P < .01, N = 4 individual experiments. (E) Comparison of mRNA levels of HSCs maintenance genes and osteopontin (Opn) from freshly sorted Nestin+ cells from TG WT and TGRac1Δ/Δ/Rac3−/− mice by real-time PCR. Data represent mean ± SD, N = 3 individual experiments, **P < .01.
Changes in bone and sinusoids associated with deletion of Rac in Nestin+ cells are associated with decreased HSC and CXCL12 and SCF expression
Specialized regions of the BM differentially harbor quiescent or cycling HSCs in an organized microenvironment.40 In particular, quiescent HSCs have been demonstrated to be localized around Nestin+ arteries19 and perisinusoidal vessels.4,9 To determine the effect on hematopoiesis of alterations in Nestin+ cells, vascular spaces and trabecular bone in TG Rac1Δ/ΔRac3−/− mice, we determined the number of immunophenotypically-defined HSCP in the marrow and spleen of these mice compared with WT mice at 8 to 14 weeks of age. Perivascular deletion of Rac in Nestin+ cells was associated with significant decrease in BM cellularity compared with control mice (supplemental Figure 4A). Analysis of BM HSC–enriched Lineage–/lowSca-1+ckit+ (LSK) cells in Rac-deleted animals showed a significant decrease in the frequency and absolute numbers of immunophenotypically-defined primitive LSK, HSC-1 (Lineage–/lowSca-1+ckit+CD150+/highCD48–) and less primitive HSC-2 (Lineage–/lowSca-1+ckit+ CD150+/lowCD48–) cells (Figure 5A). Competitive repopulation assays confirmed a significant decrease in reconstitution of HSCs in the BM of TG Rac1Δ/ΔRac3−/− (Figure 5B-C). Multilineage progenitors (MPPs) defined as Lineage–/lowSca-1+ckit+CD150–CD48–/low and HPC-1 cells (Lineage–/lowSca-1+ckit+CD150–CD48+/high) were also decreased in the marrow, although less primitive HPC-2 progenitors defined as Lineage–/lowSca-1+ckit+CD150+CD48+ were preserved when compared with TG WT animals (supplemental Figure 4B). To test HSPCs’ functional capacity, colony-forming unit (CFU) progenitor assays were performed. The total number of CFUs was significantly decreased in the BM of TG Rac1Δ/ΔRac3−/− mice vs control mice (supplemental Figure 4C). There was no difference in the number of lineage-specified CFU-M, CFU-GM, or CFU-GMM when CFUs were analyzed in Rac-deleted and TG WT animals (supplemental Figure 4D). The peripheral blood of TG Rac1Δ/ΔRac3−/− also contained significantly fewer hematopoietic progenitors compared with WT animals (supplemental Figure 4E). Cell-cycle analysis demonstrated a significant increase in the fraction of LSK cells in G0 and concomitant decrease in LSK in S/G2/M (supplemental Figure 5A-B). In contrast, Rac excision in the Nestin+ cells led to a significant increase in the absolute number of HSC-1 and HSC-2 in the spleen (Figure 5D) but no increase in LSK compared with WT mice. The number of less primitive MPP and HPC-2 were not increased, whereas the HPC-1 population was modestly but significantly increased (supplemental Figure 4F).
Rac excision in Nestin+ cells is associated with decreased HSCs and progenitors. Immunophenotypically-defined HSC (Lineage–/lowSca-1+ckit+), respectively, HSC-1 (Lineage–/lowSca-1+ckit+CD150+/highCD48–), HSC-2 (Lineage–/lowSca-1+ckit+CD150+/lowCD48–) in (A) BM. (B) Schematic representation of competitive repopulation assay. (C) 1 000 000 of BM Lineage– cells from TG Rac1Δ/Δ Rac3−/− (CD45.2) were injected into lethally-irradiated recipients (CD45.1/CD45.2) along with 500 000 BM Lineage– competitor cells (CD45.1). Percentages of donor-derived chimerism in peripheral blood (PB) are shown at 4, 8, and 12 weeks posttransplant. Data represent mean ± SD of the percentages of donor-derived cells in the PB of recipients after infusion from 1 experiment with 4 recipients for each population, ***P < .0001. (D) Immunophenotypically defined (as in [A]) HSCs in spleen. All values are mean ± SEM, *P < .05, ** P < .01, N = 6 mice from 3 independent experiments; ns, not significant. Comparison of mRNA levels of HSCs maintenance genes, (E) stem cells factor (Scf) total, (F) m220 Scf (membrane-bound isoform), and (G) Cxcl12 from freshly sorted Nestin+ cells from TG WT and TGRac1Δ/Δ/Rac3−/− mice by quantitative real-time PCR. Data represent mean ± SEM, N = 3, *P < .05.
Rac excision in Nestin+ cells is associated with decreased HSCs and progenitors. Immunophenotypically-defined HSC (Lineage–/lowSca-1+ckit+), respectively, HSC-1 (Lineage–/lowSca-1+ckit+CD150+/highCD48–), HSC-2 (Lineage–/lowSca-1+ckit+CD150+/lowCD48–) in (A) BM. (B) Schematic representation of competitive repopulation assay. (C) 1 000 000 of BM Lineage– cells from TG Rac1Δ/Δ Rac3−/− (CD45.2) were injected into lethally-irradiated recipients (CD45.1/CD45.2) along with 500 000 BM Lineage– competitor cells (CD45.1). Percentages of donor-derived chimerism in peripheral blood (PB) are shown at 4, 8, and 12 weeks posttransplant. Data represent mean ± SD of the percentages of donor-derived cells in the PB of recipients after infusion from 1 experiment with 4 recipients for each population, ***P < .0001. (D) Immunophenotypically defined (as in [A]) HSCs in spleen. All values are mean ± SEM, *P < .05, ** P < .01, N = 6 mice from 3 independent experiments; ns, not significant. Comparison of mRNA levels of HSCs maintenance genes, (E) stem cells factor (Scf) total, (F) m220 Scf (membrane-bound isoform), and (G) Cxcl12 from freshly sorted Nestin+ cells from TG WT and TGRac1Δ/Δ/Rac3−/− mice by quantitative real-time PCR. Data represent mean ± SEM, N = 3, *P < .05.
Published data have reported that Nestin+ cells, CAR cells, and LepR+ cells represent overlapping cell populations and are major sources of HSC maintenance factors, such as Scf and Cxcl12.4,18,19 Moreover, LepR+ cells are critical for the maintenance of quiescent HCSs.18 Quantitative polymerase chain reaction (PCR) analysis of freshly isolated Nestin+ cells from Rac-deleted mice compared with WT mice showed that TG Rac1Δ/ΔRac3−/− had a significant decrease in the expression of Scf, particularly the membrane-bound isoform (m220 Scf) and Cxcl12 (Figure 5E-G). In summary, changes in number of Nestin+ cells post–Rac excision, was associated with a significant change in the ratio of arterioles to sinusoids, a marked alteration in trabecular bone space with reduced numbers of HSCP, and their functions in the marrow and blood of these animals.
Discussion
The Rac-signaling pathway, including the Rac-guanine exchange factor Vav1, the effector p21–activated kinase (Pak)-2 and the Rho GTPases Rac1 and Rac2, integrates critical signals between HSCs and the HM.26,41,42 Rac proteins regulate HSC cell adhesion, cell polarity, engraftment, and cell cycle and cell proliferation (reviewed in Troeger and Williams27 ). Nestin+ stromal cells are thought to be required for maintenance of HSC.4 Here we demonstrate that Nestin+ cells require Rac activity for their growth, differentiation, and survival. Excision of Rac genes in the perivascular niche leads to a significant decrease of Nestin+ cells in vivo and an induction of cell death in vitro. These changes were associated with a marked change in vascular and bone architecture and alteration of the number of immunophenotypically and functionally defined HSCs and progenitor cells in both the medullary cavity and the spleen.
Previous studies have suggested that BM vascular compartments maintain HSCs according to vessel type.19 Sinusoids represent the most abundant vessels in the BM and colocalize with perivascular stem cells and HSCs, whereas arterioles have been implicated in maintenance of HSC quiescence.20 Here we show that Rac deletion in the perivascular niche leads to a significant loss in the number of arterioles and an increase in the sinusoidal vasculature. Previous studies using a Nestin-GFP mouse model have demonstrated nestin expression along larger vessels in the BM and in perisinusoidal stromal cells.9,20 In contrast, reports of Nes-Cre mouse models showed that nestin expression was mainly identified around larger BM vessels.9 Mendez-Ferrer et al4 have reported that Nestin+ cells contribute to the osteochondral development when ROSA26/loxP-stop-loxP-lacZ (R26R) reporter mice are crossed with the Nes-Cre transgenic line but not when crossed with the Nes-creER line. Thus, different nestin transgenic lines appear to target expression in a different subpopulation of endothelial and perivascular stromal cells in the HM, complicating interpretation of the phenotypes of these lines.23 Here we show that Nes-Cre–directed excision of Rac1 in the Rac3-null genetic background leads to significant deletion of Rac in a defined population of perivascular cells. One weakness of the approach reported here is that there is a modest deletion of Rac in endothelial cells, although the biological relevance of this deletion is unclear.
The BM vascular niche creates a unique and organized environment for HSCs. The endosteal niche is a highly vascularized compartment encompassing sinusoids and arteriolar vessels.32,43 MSCs may promote HSCs’ maintenance or proliferation depending on their localization around the blood vessels or the endosteum. Quiescent HSCs have been identified around arterioles in the marrow of long and sternal bones.19 Alternately, HSCs were also found around sinusoidal vessels and colocalize with other mesenchymal cells.9 Here we show that deletion of Rac genes in the perivascular niche is associated with depletion of HSCs from the BM. Mechanistically, deletion of Rac in the perivascular space was accompanied by a significant decrease in the expression of Scf and Cxcl12, factors implicated in HSC maintenance. Recent studies have demonstrated that LepR+ cells represent the major source of these factors in the BM.18 Thus, Scf deletion in LepR+ cells led to depletion of quiescent HSCs from the BM,9,22 whereas excision of Cxcl12 in LepR+ cells induced HSC mobilization.23 Several groups have reported a close overlap among Nestin-GFP+ perivascular cells, LepR+-expressing MSCs, and CAR cells.9,21 The data presented here are consistent with these previous findings and further support the importance of the perivascular niche in hematopoiesis. Our data reveal a phenotype characterized by an inverted ratio between the arteriolar and sinusoidal vascular compartments. Despite the increase in sinusoidal space and medullary bone formation that we observed in this model, we did not observe an increase in HSC frequency.
We have previously shown that deletion of Rac genes in osteoblasts resulted in a significant reduction of trabecular bone volume and abnormal bone architecture.2 In contrast, Rac excision in the Nestin+ cells results in an increase in trabecular and cortical bone formation and an increase in the number of osteoblast cells in vivo. The endosteal MSCs reside in the perivascular region of the BM and migrate to the endosteal surface to differentiate in osteoblasts.3,32,43 Our results suggest that the deletion of Rac genes in the Nestin+ cells affects their differentiation into bone-forming progenitors. Increased trabecular bone formation in TG Rac1Δ/ΔRac3−/− mice was associated with increased RNA expression of the osteoblast markers osteopontin, alkaline phosphatase, and Runx2 in isolated Nestin+ cells. Despite the significant increase in trabecular bone in TG Rac1Δ/ΔRac3−/− mice, there was a decrease in HSC frequency in these mice, suggesting that the amount of trabecular bone does not correlate directly with maintenance of these cells in vivo.
In conclusion, Rac GTPase activity appears important for the survival and differentiation of Nestin+ cells in the perivascular space. Rac signaling within the perivascular niche and endothelial cells appears to be critical to the integrity of arteriolar and sinus vascular structures and maintenance of hematopoiesis. The present study provides new information regarding the role of Rac signaling in the BM perivascular niche and further supports the importance of Rac regulation of HSCs and HM constituents and confirms the complexity of the HM with respect to the contribution of different cell types to the HSC niche.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of our laboratory, Steve Lane, David Scadden, and Reinhard Henschler for helpful discussions; Maria Suarez and Natasha Rossi for administrative support; Meaghan McGuinness and Chad Harris for technical assistance; and Rebekka Schneider-Karamann for IHC staining.
This work is supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant DK062757 (D.A.W.), National Heart, Lung, and Blood Institute grants R01 HL093139 (L.E.S.) and P01 HL095489 (L.E.S.), and Day Kimball Healthcare grant D/12/03783 (M.F.C.).
Authorship
Contribution: M.F.C., S.-Y.P., K.C., and R.M. performed the experiments; M.F.C. and S.-Y.P. drafted the paper; and L.E.S. and D.A.W. supervised the experiments and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Williams, 300 Longwood Ave, Karp 08125.3, Boston, MA 02115; e-mail: dawilliams@childrens.harvard.edu.

![Figure 2. Rac deletion in Nestin+ cells disturbs the vascular morphology in the medullary cavity. (A) Snapshot images of 3D reconstructed whole-mount sections showing BM microvasculature in the endosteal region of the femoral diaphysis from each genotype after staining for laminin (green, arteries and sinusoids), endoglin (red, sinusoids), and Sca-1 (blue, arteries) as detailed in Material and methods. (B) Sinusoidal volume and (C) arteriolar volume quantification of diaphysis from snapshot images of 3D-reconstructed whole-mount sections (as shown in [A]) was determined using Volocity 3D imaging software. Data represent mean ± standard error of the mean (SEM), N = 4 animals per group, *P < .05, 4-5 individual sections analyzed per mouse. (D) Changes in sinusoidal architecture as assessed LSC of whole longitudinal image of femoral cryosection stained with DAPI (nuclei) showing proximal metaphysis (PM), diaphysis (DIA), and distal metaphysis (DM). Representative LSC images (middle panels) of BM stained for endoglin (red), laminin (green), and DAPI (blue) of TG WT (top panel) and TG Rac1 Δ/ΔRac3−/− mice (bottom panel). The middle panel shows high-resolution images of the indicated field. The right panel show images of contoured niche signals of indicated fields. Analysis of endoglin+ signal events as detailed in Material and methods, based on fluorescence intensity (as shown in supplemental Figure 2A-B) and detected by the iCys software (teal marks). (E) Quantitative analysis of the frequency of sinusoidal distribution by diameter of BM vessels of images in (D, middle panels) determined using iCys software, in a blinded manner. Data represent mean ± SEM, N = 3 animals per group, *P < .05, **P < .01. (F) Vascular sinusoidal niche area quantification of diaphysis from photographs in (D, right panel) was assessed using iCys software. Data represent mean ± SEM, N = 3 animals per group, *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/20/10.1182_blood-2014-10-604892/4/m_3105f2.jpeg?Expires=1770155046&Signature=jZ7zQwg9HvmrVR6D3xKI72D90p6wLIWp36OQXFAugIHA5nPX-e8SNbS8eNP3Rbg0svJrG8s3YSF~6rhpxSko8W-rTMES65qDoh7c1sMcb6zBEVcQX8v6rnNt5DUgUbBA2hlk65dbuWm7Gh4CM1R~pLTaeJpa-MCqZP-o~oki00FSEp6M-bJlC~c-Ne0C3eIX-5e7DXnFjIvZdBBsZM~VPPuZn6iBMVmKBlCVVoLkof3G1pOZvPBnhBSGYIyeq0uRhLcwzN9voo8~LN3ubBj45ImgQy5fEOX5hdDUSOJztvw-AFI22Ll1qJS13Q4lYkewPKyf4puVVCkkFir-vttL5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Rac excision in Nestin+ cells is associated with decreased HSCs and progenitors. Immunophenotypically-defined HSC (Lineage–/lowSca-1+ckit+), respectively, HSC-1 (Lineage–/lowSca-1+ckit+CD150+/highCD48–), HSC-2 (Lineage–/lowSca-1+ckit+CD150+/lowCD48–) in (A) BM. (B) Schematic representation of competitive repopulation assay. (C) 1 000 000 of BM Lineage– cells from TG Rac1Δ/Δ Rac3−/− (CD45.2) were injected into lethally-irradiated recipients (CD45.1/CD45.2) along with 500 000 BM Lineage– competitor cells (CD45.1). Percentages of donor-derived chimerism in peripheral blood (PB) are shown at 4, 8, and 12 weeks posttransplant. Data represent mean ± SD of the percentages of donor-derived cells in the PB of recipients after infusion from 1 experiment with 4 recipients for each population, ***P < .0001. (D) Immunophenotypically defined (as in [A]) HSCs in spleen. All values are mean ± SEM, *P < .05, ** P < .01, N = 6 mice from 3 independent experiments; ns, not significant. Comparison of mRNA levels of HSCs maintenance genes, (E) stem cells factor (Scf) total, (F) m220 Scf (membrane-bound isoform), and (G) Cxcl12 from freshly sorted Nestin+ cells from TG WT and TGRac1Δ/Δ/Rac3−/− mice by quantitative real-time PCR. Data represent mean ± SEM, N = 3, *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/20/10.1182_blood-2014-10-604892/4/m_3105f5.jpeg?Expires=1770155046&Signature=d7EjAXcz0prk3Mhj1nznkEt3tqHSM6Q2bTicPUZ-zwunTdxJi1xeNHYEycPwDAlYxt6vXOOrcHayvDKLWU3VWj8ooIRaXRkHQehgqxtZX0WCLqbhPzKLspr1ZTZnoTw36~1fRZSFIZNtL2huYCKo-LU2V5RMKkY2GNzmwXrSSe0TT0rb33xgNbrwKiu~Lno8O0RYEgJM2FJEK4Zxfdad91B8BWccYyT6tfU2Z3viWhGinMSo4J8O4p8osthi~8I9Us2wgOOM6ICvfuq3KMPWRLte69iU6Ah6wibHMo4GYEd5G-UaKlxvvFI744gngX18~sue7SKVwnpibrTJkDc0KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Rac deletion in Nestin+ cells disturbs the vascular morphology in the medullary cavity. (A) Snapshot images of 3D reconstructed whole-mount sections showing BM microvasculature in the endosteal region of the femoral diaphysis from each genotype after staining for laminin (green, arteries and sinusoids), endoglin (red, sinusoids), and Sca-1 (blue, arteries) as detailed in Material and methods. (B) Sinusoidal volume and (C) arteriolar volume quantification of diaphysis from snapshot images of 3D-reconstructed whole-mount sections (as shown in [A]) was determined using Volocity 3D imaging software. Data represent mean ± standard error of the mean (SEM), N = 4 animals per group, *P < .05, 4-5 individual sections analyzed per mouse. (D) Changes in sinusoidal architecture as assessed LSC of whole longitudinal image of femoral cryosection stained with DAPI (nuclei) showing proximal metaphysis (PM), diaphysis (DIA), and distal metaphysis (DM). Representative LSC images (middle panels) of BM stained for endoglin (red), laminin (green), and DAPI (blue) of TG WT (top panel) and TG Rac1 Δ/ΔRac3−/− mice (bottom panel). The middle panel shows high-resolution images of the indicated field. The right panel show images of contoured niche signals of indicated fields. Analysis of endoglin+ signal events as detailed in Material and methods, based on fluorescence intensity (as shown in supplemental Figure 2A-B) and detected by the iCys software (teal marks). (E) Quantitative analysis of the frequency of sinusoidal distribution by diameter of BM vessels of images in (D, middle panels) determined using iCys software, in a blinded manner. Data represent mean ± SEM, N = 3 animals per group, *P < .05, **P < .01. (F) Vascular sinusoidal niche area quantification of diaphysis from photographs in (D, right panel) was assessed using iCys software. Data represent mean ± SEM, N = 3 animals per group, *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/20/10.1182_blood-2014-10-604892/4/m_3105f2.jpeg?Expires=1770155047&Signature=mTVK4C5DpdGWttuRVZq6JFr6XqMKsxk9u9oNvVWjUfQM8zc7vND4x5txR19FR-h4OnsFW336i1T6FabKW9gbQUQZ9wd7GqPIkVPWULZ3zGZ5mXktx9XcAqEgdLzMVk36XM2z4YPPHLZYpqgaFnlH33jxpE9knAIKFNWKmfBvYOTxC9Yoep664wVQ8fzjHMjLdr1Cf59J7RJh8B5HY-y7GPiPkoRvuazwl9MEFyJMGytcNlPytLTnhEBDQY~ikLXxv27hcdGU8BXdEvWBbYgOea7Y06SEF5cZhq4JRJL63pJ36zZNmgmrxk3A3VYMCgphBFf5outdLPVXgHLudqV2cQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Rac excision in Nestin+ cells is associated with decreased HSCs and progenitors. Immunophenotypically-defined HSC (Lineage–/lowSca-1+ckit+), respectively, HSC-1 (Lineage–/lowSca-1+ckit+CD150+/highCD48–), HSC-2 (Lineage–/lowSca-1+ckit+CD150+/lowCD48–) in (A) BM. (B) Schematic representation of competitive repopulation assay. (C) 1 000 000 of BM Lineage– cells from TG Rac1Δ/Δ Rac3−/− (CD45.2) were injected into lethally-irradiated recipients (CD45.1/CD45.2) along with 500 000 BM Lineage– competitor cells (CD45.1). Percentages of donor-derived chimerism in peripheral blood (PB) are shown at 4, 8, and 12 weeks posttransplant. Data represent mean ± SD of the percentages of donor-derived cells in the PB of recipients after infusion from 1 experiment with 4 recipients for each population, ***P < .0001. (D) Immunophenotypically defined (as in [A]) HSCs in spleen. All values are mean ± SEM, *P < .05, ** P < .01, N = 6 mice from 3 independent experiments; ns, not significant. Comparison of mRNA levels of HSCs maintenance genes, (E) stem cells factor (Scf) total, (F) m220 Scf (membrane-bound isoform), and (G) Cxcl12 from freshly sorted Nestin+ cells from TG WT and TGRac1Δ/Δ/Rac3−/− mice by quantitative real-time PCR. Data represent mean ± SEM, N = 3, *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/20/10.1182_blood-2014-10-604892/4/m_3105f5.jpeg?Expires=1770155047&Signature=dT3Un~3nGgoOh6bCF3SFlgEeruI3s7OaFXfZrtn6pextMrjnRaOzwcdZemkaJTuAIwq8GQTPMnIi7njIOMBMmKb2-zoMI1clHDFlFJf4eZC9C~vaZKOr~5cp26AJ8IwrjMIr8dWZ7sJJmg5ptF-mWEa4F7iuJQMNYpG8rnuDqopORc~O474PNrGN0~klIdmGcQnCI92BCvyooHaXJtwv-5bShhmev7n3AFxb9Fso997YAwAAcCRz0fNuz7Sz5wp1MpEuQBPkkTSb2fPnIK8tIuWae4SBtz28XAvidGDJ0aC1wvS449KWbs6rUGe8VJpYIGAs4VdU99Pw9EB152fZpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)